
3 курс / Фармакология / Essential_Psychopharmacology_2nd_edition
.pdf
CHAPTER 6
CLASSICAL ANTIDEPRESSANTS,
SEROTONIN SELECTIVE REUPTAKE
INHIBITORS, AND NORADRENERGIC
REUPTAKE INHIBITORS
I.Theories of antidepressant drug action
A.Classifications based on acute pharmacologic actions
B.The neurotransmitter receptor hypothesis of antidepressant action
C.The monoamine hypothesis of antidepressant action on gene expression II. Pharmacokinetics of antidepressants
A.CYP450 1A2
B.CYP450 2D6
C.CYP450 3A4
D.CYP450 inducers III. Classical antidepressants
A.Monoamine oxidase inhibitors
B.Tricyclic antidepressants
IV. Selective serotonin reuptake inhibitors
A.What five drugs share in common
B.Pharmacologic and molecular mechanisms of action of the SSRIs
C.Serotonin pathways and receptors that mediate therapeutic actions and side effects of SSRIs
D.Not-so-selective serotonin reuptake inhibitors: five unique drugs or one class with five members?
V. Selective noradrenergic reuptake inhibitors VI.
Norepinephrine and dopamine reuptake blockers VII.
Summary
In this chapter, we will review pharmacological concepts underlying the use of several classes of antidepressant drugs, including the classical monoamine oxidase (MAO) inhibitors, the classical tricyclic antidepressants, the popular serotonin selective reuptake inhibitors (SSRIs), and the new selective noradrenergic reuptake
199
200Essential Psychopharmacology
inhibitors, as well as norepinephrine and dopamine reuptake inhibitors. The goal of this chapter is to acquaint the reader with current ideas about how these antidepressants work. We will explain the mechanisms of action of these drugs by building on general pharmacological concepts. We will also introduce pharmacokinetic concepts for the antidepressants, namely, how the body acts on these drugs through the cytochrome P450 enzyme system.
Our treatment of antidepressants in this chapter is at the conceptual level and not at the pragmatic level. The reader should consult standard drug handbooks for details of doses, side effects, drug interactions, and other issues relevant to the prescribing of these drugs in clinical practice.
Theories of Antidepressant Drug Action
Classifications Based on Acute Pharmacological Actions
We do not currently have a complete and adequate explanation of how antidepressant drugs work. What we do know is that all effective antidepressants have identifiable immediate interactions with one or more monoamine neurotransmitter receptor or enzyme. These immediate actions provide the pharmacological foundation for the current classification of the different antidepressants.
According to this classification scheme, there are at least eight separate pharmacological mechanisms of action and more than two dozen antidepressants. Most antidepressants block monoamine reuptake, but some block alpha 2 receptors and others the enzyme monoamine oxidase (MAO). Some antidepressants have direct actions on only one monoamine neurotransmitter system; others have direct actions on more than one monoamine neurotransmitter system. As discussed in Chapter 5, the immediate pharmacological actions of all antidepressants eventually have the effect of boosting the levels of monoamine neurotransmitters (Figs. 5 — 15 and 5 — 16; see also Fig. 6—1). This chapter and the following chapter will review those specific receptors and enzymes that are influenced by each of the various antidepressants immediately after administration to a depressed patient. Just how all these different immediate pharmacological actions result ultimately in an antidepressant response a few weeks after administration of an antidepressant agent—that is, the final common pathway of antidepressant treatment response—is the subject of intense research interest and debate (Fig. 6—1). Currently, there is intense focus on the gene expression that is triggered by antidepressants. The monoamine hypothesis of antidepressant action on gene expression suggests that gene expression is ultimately the most important action of antidepressants.
The Neurotransmitter Receptor Hypothesis of Antidepressant Action
One theory to explain the ultimate mechanism of delayed therapeutic action of antidepressants is the neurotransmitter receptor hypothesis of antidepressant action (Figs. 6—1 through 6—6). This is a hypothesis related to the neurotransmitter receptor hypothesis of depression discussed in Chapter 5 (Figs. 5—60 through 5— 62). As previously discussed, this latter hypothesis proposes that depression itself is linked to abnormal functioning of neurotransmitter receptors.

Classical Antidepressants, Serotonin Selective and Noradrenergic Reuptake Inhibitors |
201 |
FIGURE 6—1. This figure depicts the different time courses for three effects of antidepressant drugs, namely clinical changes, neurotransmitter (NT) changes, and receptor sensitivity changes. Specifically, the amount of NT changes relatively rapidly after an antidepressant is introduced. However, the clinical effect is delayed, as is the desensitization, or down regulation, of neurotransmitter receptors. This temporal correlation of clinical effects with changes in receptor sensitivity has given rise to the hypothesis that changes in neurotransmitter receptor sensitivity may actually mediate the clinical effects of antidepressant drugs. These clinical effects include not only antidepressant and anxiolytic actions but also the development of tolerance to the acute side effects of antidepressant drugs.
Whether or not neurotransmitter receptors are abnormal in depression, the neurotransmitter receptor hypothesis of antidepressant action proposes that antidepressants, no matter what their initial actions on receptors and enzymes, eventually cause a desensitization, or down regulation, of key neurotransmitter receptors in a time course consistent with the delayed onset of antidepressant action of these drugs (Figs. 6—1 through 6 — 6).
This time course coincides with other events, including the time it takes for a patient to become tolerant to the side effects of antidepressants. Thus, desensitization of some neurotransmitter receptors may lead to the delayed therapeutic actions of antidepressants, whereas desensitization of other neurotransmitter receptors may lead to the decrease of side effects over time.
An overly simplistic view of the neurotransmitter receptor hypothesis of depression is that the normal state becomes one of depression as neurotransmitter is depleted and postsynaptic receptors then up-regulate (Fig. 6—2). Boosting neurotransmitters by MAO inhibition (Figs. 6—3 and 6—4) or by blocking reuptake pumps for monoamine neurotransmitters (Figs. 6—5 and 6—6) eventually results in the down regulation of neurotransmitter receptors in a delayed time course more closely related to the timing of recovery from depression (Figs. 6—1, 6—4, and 6—6).
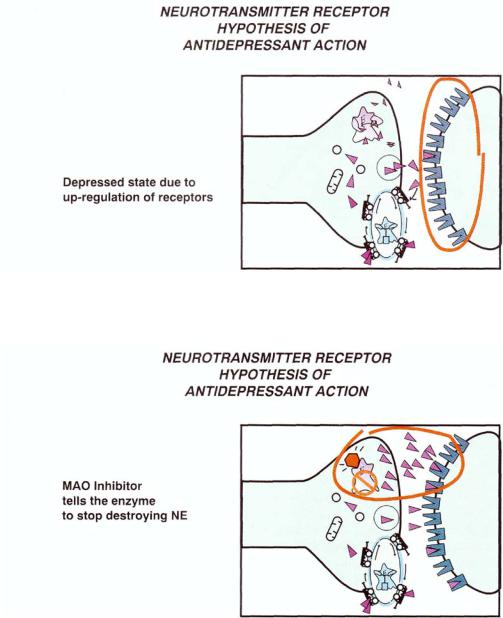
202 Essential Psychopharmacology
FIGURE 6—2. The neurotransmitter receptor hypothesis of antidepressant action—part 1. Shown here is the monoaminergic neuron in the depressed state, with up regulation of receptors (indicated in the red circle).
FIGURE 6—3. The neurotransmitter receptor hypothesis of antidepressant action—part 2. Here, a monoamine oxidase (MAO) inhibitor is blocking the enzyme and thereby stopping the destruction of neurotransmitter. This causes more neurotransmitter to be available in the synapse (indicated in the red circle).
Originally, it was hypothesized that desensitization of postsynaptic receptors may be responsible for the therapeutic actions of antidepressants. It is now clear that desensitization of some postsynaptic receptors is responsible for the development of tolerance to the acute side effects of antidepressants. Attention is currently being

Classical Antidepressants, Serotonin Selective and Noradrenergic Reuptake Inhibitors |
203 |
FIGURE 6—4. The neurotransmitter receptor hypothesis of antidepressant action—part 3. The consequence of long-lasting blockade of monoamine oxidase (MAO) by an MAO inhibitor is that the neurotransmitter receptors are desensitized or down-regulated (indicated in the red circle).
FIGURE 6—5. The neurotransmitter receptor hypothesis of antidepressant action—part 4. Here, a tricyclic antidepressant blocks the reuptake pump, causing more neurotransmitter to be available in the synapse (indicated in the red circle). This is very similar to what happens after MAO is inhibited (Fig. 6-3).
focused on the presynaptic receptors and their desensitization in order to explain the therapeutic actions of antidepressants. This will be discussed in more detail in the section on serotonin selective reuptake inhibitors (SSRIs).
The Monoamine Hypothesis of Antidepressant Action on Gene Expression
As discussed in Chapter 5 (Figs. 5—63 and 5—64), the monoamine hypothesis of gene expression proposes that depression itself is linked to abnormal functioning of neurotransmitter-inducible gene expression, particularly neurotrophic factors such as

204 Essential Psychopharmacology
FIGURE 6—6. The neurotransmitter receptor hypothesis of antidepressant action—part 5. The consequence of long-lasting blockade of the reuptake pump by a tricyclic antidepressant is to cause the neurotransmitter receptors to become desensitized or down-regulated (indicated in the red circle). This is the same outcome as with long-lasting blockade of MAO (see Fig. 6—4).
brain-derived neurotrophic factor (BDNF), leading to atrophy and apoptosis of critical hippocampal neurons. Whether or not the transduction of a monoaminergic neuronal impulse into gene expression is actually abnormal in depression, the monoamine hypothesis of antidepressant action on gene expression proposes that antide-pressants, no matter what their initial actions on receptors and enzymes, eventually cause critical genes to be activated or inactivated. One of these may indeed be BDNF, although many others are undoubtedly involved as well (Fig. 6 — 7). Changes in the genetic expression of monoamine neurotransmitter receptors have already been discussed (Figs. 6—1 through 6—6). Thus, the gene expression hypothesis is consistent with the monoamine receptor hypothesis of antidepressant action but is broader in scope.
Delayed actions of antidepressants may not only explain the delay in onset of therapeutic action of antidepressants; they may also explain why some patients fail to respond to antidepressants, since it is possible that in such patients the initial pharmacological actions are not translated into the required delayed pharmacologic and genetic actions. Knowing the biological basis for treatment nonresponse may lead to a greatly needed advance in the pharmacotherapy of depression, namely an effective treatment for refractory or nonresponding depressed patients, as discussed in Chapter 5. Also, if one understands the key pharmacologic events that are linked to the therapeutic actions of the drugs, it may be possible to accelerate them with future drugs. If so, it could lead to another highly desired advance in the pharmacotherapy of depression, namely a rapid-onset antidepressant.
In summary, all antidepressants have a common action on monoamine neurotrans- mitters—they boost monoamine neurotransmission, leading to changes in gene expression in the neurons targeted by the monoamines. This includes desensitization of neurotransmitter receptors, leading to both therapeutic action and tolerance to side effects. Although antidepressants are classified on the basis of those actions on neurotransmitter receptors and enzymes that are immediate, attention is increasingly being paid to how these initial and immediate actions translate into delayed actions.

Classical Antidepressants, Serotonin Selective and Noradrenergic Reuptake Inhibitors |
205 |
FIGURE 6-7. The monoamine hypothesis of antidepressant action on gene expression is shown here. The neurotransmitter at the top is presumably increased by an antidepressant. The cascading consequence of this is ultimately to change the expression of critical genes in order to effect an antidepressant response. This includes down-regulating some genes so that there is decreased synthesis of receptors, as well as up-regulating other genes so that there is increased synthesis of critical proteins, such as brain-derived neurotrophic factor (BDNF).
Pharmacokinetics of Antidepressants
Recently, there has been a rapid increase in our knowledge about how antidepressants and mood stabilizers are metabolized and about drug interactions with antidepressants and mood stabilizers. Pharmacokinetics is the study of how the body acts on drugs, especially to absorb, distribute, metabolize, and excrete them. These phar-macokinetic actions are mediated through the hepatic and gut drugmetabolizing system known as the cytochrome P450 (CYP450) enzyme system.
The CYP450 enzymes and the pharmacokinetic actions they represent must be contrasted with the pharmacodynamic actions of antidepressants, which were discussed in the previous section on the mechanism of action of antidepressants. Although

206 Essential Psychopharmacology
Table 6—1. Pharmacokinetics and pharmacodynamics
PHARMACOKINETICS:
How the body acts on drugs
PHARMACODYNAMICS:
How drugs act on the body, especially the brain
FIGURE 6—8. A drug is absorbed and delivered through the gut wall to the liver to be biotransformed so that it can be excreted. Specifically, the cytochrome P450 (CYP450) enzyme in the gut wall or liver converts the drug substrate into a biotransformed product in the bloodstream. After passing through the gut wall and liver (left), the drug will exist partly as unchanged drug and partly as biotransformed drug (right).
most of this book deals with the pharmacodynamics of psychopharmacological agents, especially how these drugs act on the brain, the following section will discuss the pbarmacokinetics of antidepressants and mood stabilizers, or how the body acts on these drugs (Table 6—1).
The CYP450 enzymes follow the principle of transforming substrates into products. Figure 6 — 8 shows how an antidepressant is absorbed and delivered through the gut wall to the liver to be biotransformed so that it can be excreted from the body. Specifically, the CYP450 enzyme in the gut wall or liver converts the drug substrate into a biotransformed product in the bloodstream. After passing through the gut wall and liver, the drug will exist partly as unchanged drug and partly as biotransformed product (Fig. 6—8).
There are several known CYP450 systems. Five of the most important enzymes for antidepressant drug metabolism are shown in Figure 6—9. There are over 30

FIGURE 6—9. There are several known CYP450 enzyme systems. Five of the most important for antidepressant and mood stabilizer metabolism are shown here.
FIGURE 6—10. Not all individuals have the same CYP450 enzymes. For example, about 1 in 20 Caucausians is a poor metabolizer via 2D6 and must metabolize drugs by an alternative route, which may not be as efficient.
207

208 Essential Psychopharmacology
FIGURE 6—11. Certain tricyclic antidepressants, especially secondary amines such as clomipramine and imipramine, are substrates for CYP450 1A2. This enzyme converts the tricyclics into active metabolites by demethylation to form desmethylclomipramine and desipramine, respectively.
known CYP450 enzymes, and probably many more awaiting discovery and classification. Not all individuals have all the same CYP450 enzymes. In such cases, the enzyme is said to be polymorphic. For example, about 5 to 10% of Caucasians are poor metabolizers via the enzyme CYP450 2D6 (Fig. 6—10). They must metabolize drugs by alternative routes, which may not be as efficient as the CYP450 2D6 route. Another CYP450 enzyme, 2C19, has reduced activity in approximately 20% of Japanese and Chinese individuals and in 3 to 5% of Caucasians.
CYP450 1A2
One CYP450 enzyme of relevance to antidepressants is 1A2 (Figs. 6—11 and 6—12). Certain tricyclic antidepressants (TCAs) are substrates for this enzyme, especially the secondary amines such as clomipramine and imipramine (Fig. 6—11). CYP450 1A2 demethylates such TCAs, but does not thereby inactivate them. In these cases, the desmethyl metabolite of the TCA (e.g., desmethylclomipramine and desipramine) is still an active drug (Fig. 6—12).
CYP450 1A2 is inhibitedhy the serotonin selective reuptake inhibitor fluvoxamine (Fig. 6—12) Thus, when fluvoxamine is given concomitantly with other drugs that use 1A2 for their metabolism, those drugs can no longer be metabolized as efficiently.
