
151
Batyrenko, A. M. Malkova, V. V. Sharoyko, K. N. Semenov ‘Synthesis and study of the biological activity of new derivatives 1 ,3,5-triazine’, Sochi, Russian Federation, 2021.
6. Materials of the XXVIII All-Russian Conference of Young Scientists with International Participation ‘Actual Problems of Biomedicine — 2022’ March 24–26, 2022. O. V. Mikolaichuk, E. A. Popova, A. V. Protas, M. D. Luttsev, A. A. Potanin, A. M. Malkova
‘Study of the biocompatibility and bioactivity of a new tetrazole-containing derivative of 2-amino-4,6 -di(aziridin-1-yl)-1,3,5-triazine’, St. Petersburg, Russian Federation, 2022.
The work was supported financially by the Ministry of Health of the Russian Federation (government assignment on the topic ‘Creation and evaluation of the antitumour activity of conjugates of non-annulated 1,3,5-triazinyltetrazoles with targeted delivery molecules to microenvironment tumour cells targets’).
Provisions for defense
1.Method for the synthesis of the cytostatic drug 5-((4,6-dichloro-1,3,5-triazin-2- yl)amino)-2,2-dimethyl-1,3-dioxan-5-yl)methanol and its tetrazolated derivative ((5-((4,6- di(aziridin-1-yl)-1,3,5-triazin-2-yl)amino)-2,2-dimethyl-1,3-dioxan-5-yl)methyl-2-(5- phenyl-2H-tetrazol-2-yl)acetate).
2.Data on the synthesis and identification of graphene oxide conjugate with a cytostatic preparation based on 1,3,5-triazine.
3.Study of the properties of aqueous solutions of 5-((4,6-dichloro-1,3,5-triazin-2- yl)amino)-2,2-dimethyl-1,3-dioxan-5-yl)methanol and its tetrazolated analogue.
4.Results of the study of biocompatibility and biological activity of 1,3,5-triazine derivatives and a conjugate based on it.
Thesis structure
The dissertation work is presented on 137 pages and consists of a list of used abbreviations, an introduction, a literature review, a discussion of the results, conclusions, an experimental part and a list of references. The list of cited literature includes 133 titles. The dissertation contains 23 tables, 63 figures and 31 schemes.
The personal contribution of the author consisted in the synthesis and identification of derivatives of 1,3,5-triazine and triazinyltetrazoles, graphene oxide and conjugate based on it, in the study of biocompatibility and biological activity of the obtained compounds and conjugate, discussion of experimental results and preparation of scientific publications.
152
Acknowledgment
The author expresses his gratitude to everyone with whom the appearance of this work was connected, first of all to his supervisors, Dr Sci., Associate Professor, Head of the Department of General and Bioorganic Chemistry, Pavlov First St. Petersburg State Medical University Semenov Konstantin Nikolaevich and Dr Sci., Associate Professor, Professor of the Department Sharoyko Vladimir Vladimirovich, the staff of the department: Dr Sci., Professor Popova Elena Alexandrovna, PhD, Associate Professor Protas Alexandra Vladimirovna, Shemchuk Olga Sergeevna. The author thanks the staff of the Department of Solid-State Chemistry, Institute of Chemistry, St. Petersburg State University, for organising the work at a high level and creating a creative atmosphere, headed by Dr Sci., Professor Murin Igor Vasilyevich. The author thanks the staff of the Department of Chemistry and Technology of Organic Nitrogen Compounds of the St. Petersburg State Technical University, Dr Sci., Professor Ostrovsky Vladimir Aronovich for advice and scientific consultations.
The author also expresses his gratitude to I. V. Kornyakov for help with experiments with X-ray diffraction data, M. D. Lutsev for help in conducting biocompatibility studies. The author also expresses his gratitude to all the staff of the Resource Centre of SaintPetersburg University for recording NMR, IR, mass spectra and phisical and chemical study.
Рекомендовано к изучению сайтом МедУнивер - https://meduniver.com/

153
CHAPTER 1. LITERATURE REVIEW
1. METHODS FOR THE SYNTHESIS OF TRI-SUBSTITUTED 1,3,5-
TRIAZINES WITH ANTI-TUMOUR ACTIVITY
From a wide range of substituted triazines, 1,3,5-triazines stand out due to their unique properties [2]. Many compounds containing 1,3,5-triazine in their structure exhibit a wide range of biological activity, which stimulates the development of new methods and original approaches to the synthesis of trisubstituted triazines [3–5]. This literature review considers the most important methods for the synthesis of substituted 1,3,5-triazines. The main synthetic approaches to the preparation of substituted 1,3,5-triazines are the cyclisation reactions of aldehydes, nitriles, biguanides and the reactions of successive substitution of chlorine atoms in cyanuric chloride.
1.1.Cyclisation reactions
1.1.1.Obtaining trisubstituted 1,3,5-triazines by the cyclisation reaction of
biguanide derivatives
Arylbiguanides can participate in two types of heterocyclisation reactions with the formation of triazines at fragment “A” (with the participation of aromatic acid esters, esters of monochloroacetic acid) and at fragment “B” (with the participation of acetoacetic ester and β-diketones) with the formation of pyrimidine derivatives (Fig. 1.1).
Fig. 1.1. General formula of arylbiguanides.
Thus, in [6], the interaction of esters (1.1–1.3) with the corresponding biguanide hydrochlorides in a solution of sodium methoxide in methanol (MeONa/MeOH) at reflux for 45 h gave the series of 2-[(4-amino-6-R2-1,3,5-triazin-2-yl)methylthio]-N-(1-R1- imidazolidin-2-ylidene)-4-chloro-5-methylbenzenesulphonamides (1.4–1.6; Scheme 1.1).

154
Scheme 1.1
The effect of compounds 1.4–1.6 on the viability of three tumour cell lines HCT116 (human intestinal carcinoma), MCF-7 (human breast ductal adenocarcinoma), and HeLa (human cervical adenocarcinoma), as well as cells of non-tumour origin — HaCaT (keratinocytes) was studied using the MTT test. The authors of [6] determined IC50 after 72 h of cell incubation; cisplatin was used as the reference drug. MTT results: compound 1.4 (R1 = benzyl, R2 = indoline) — IC50 = 6 µM for HCT-116, IC50 = 7 µM for MCF-7; compound 1.5 (R1 = 4-trifluoromethylbenzyl, R2 = 4-benzylpiperazine) — IC50 = 5 μM for
HCT-116, IC50 = 8 μM for HeLa; compound 1.6 (R1 = 4-trifluoromethylbenzyl, R2 = chlorine) — IC50 = 5 μM for HCT-116, IC50 = 6 μM for MCF-7. The IC50 values of compounds 1.4–1.6 with respect to the HaCaT cell line were 33, 22, and 21 μM, respectively.
In another work, 1,3,5-triazine derivatives of metformin (1.7–1.9) were synthesised by condensation between biguanide and various esters [7]. The authors carried out the synthesis without isolation and purification of intermediates using biguanide hydrochloride, sodium methoxide (NaOMe) in methanol (Scheme 1.2).
Рекомендовано к изучению сайтом МедУнивер - https://meduniver.com/

155
Scheme 1.2
Next, the in vitro cytotoxic activity of compounds 1.7–1.9 against two human intestinal carcinoma cell lines (HCT-116 and SW620) was evaluated. All synthesised 1,3,5-triazine derivatives showed comparable or higher cytotoxicity compared to cisplatin. Compound 1.7 showed cytotoxicity with IC50 = 59.1 ± 1.5 μM for the SW620 cell line and
59.4 ± 1.7 μM for the HCT-116 cell line. On the contrary, the cytostatic effect of compounds 1.8 and 1.9 was very low and amounted to no more than 5–20 %. Further, in [7] a nanoform of compound 1.7 encapsulated in calcium citrate nanoparticles (1.7a) was synthesised (Scheme 1.3).
Scheme 1.3
The authors proposed a synthesis method using the binary solvent EtOH-water. Addition of 1,3,5-triazine 1.7 to an aqueous ethanol solution of calcium chloride and sodium citrate in air with vigorous stirring at room temperature led to the formation of nanoparticles 1.7a [6].
When developing nanomaterials used for targeted drug delivery, it is important to study the controlled release of a drug under physiological conditions. Both anaerobic and aerobic glycolysis lead to the accumulation of H+ in the extracellular space of tumours, causing pronounced acidification of the intercellular fluid in the tumour microenvironment [8], so the release of compound 1.7 was studied in an acidic medium at pH 5.0 and 3.0 and in a neutral medium (pH 7.4) for comparison. An in vitro study showed that 1.7a nanoparticles responded to changes in the pH of the medium with controlled release of triazine 1.7. After 24 h, 80.0 %, 48.8 %, and 30.0 % of compound 1.7 were released at pH 3.0, pH 5.0, and pH 7.4, respectively.

156
Another successful example of the synthesis of biologically active 1,3,5-triazines from biguanide derivatives is the study by Kothayer et al. [9]. The synthesis of 4-amino- 6-(arylamino)-1,3,5-triazine-2-carbohydrazide derivatives was carried out in two stages from arylbiguanide hydrochloride salts. At the first stage, salt 1.8 was neutralised using sodium methoxide in methanol, followed by its reaction with dimethyl oxalate in boiling methanol. This led to the formation of intermediate methyl 4-amino-6-(arylamino)-1,3,5- triazine-2-carboxylates (1.9–1.11) in 83–92% yield after recrystallisation from methanol. The reaction of intermediates (1.9–1.11) with hydrazine hydrate in refluxing ethanol gave the target 2,4,6-trisubstituted-1,3,5-triazines (1.12–1.14) (Scheme 1.4).
Scheme 1.4
The study of the cytotoxicity of the synthesised 4-amino-6-(arylamino)-1,3,5- triazine-2-carbohydrazides (1.12–1.14) in the concentration range of 0–125 μM was carried out on seven human tumour cell lines: ovarian adenocarcinoma (OV90), ovarian carcinomas (A2780), lung adenocarcinomas (A549), lung carcinomas (H1299), breast adenocarcinomas (MCF-7 and MDA-MB-231), and colon adenocarcinomas (HT29). Summarised data with IC50 values are presented in Table 1.1. Analysis of data on the cytotoxicity of compounds 1.12–1.14 shows that their activity is comparable to that of compound 1.15, which is an inhibitor of the ubiquitin-conjugating enzyme E2B (Rad6B;
Fig. 1.2). Rad6B activates the cell adhesion protein β-catenin, which is responsible for the malignant progression of breast cancer cells.
Рекомендовано к изучению сайтом МедУнивер - https://meduniver.com/

157
Table 1.1. IC50 values (µM) of substituted 4-amino-6-(arylamino)-1,3,5-triazine-2- carbohydrazide derivatives.
No. |
OV90 |
A2780 |
MCF-7 |
MDA- |
A549 |
H1299 |
HT-29 |
|
|
|
|
MB231 |
|
|
|
|
|
|
|
|
|
|
|
1.12 |
75.0 |
7.3 |
5.9 |
2.6 |
7.8 |
65.0 |
3.3 |
1.13 |
80.0 |
14.7 |
6.2 |
7.6 |
5.2 |
65.0 |
5.4 |
1.14 |
85.0 |
36.6 |
8.8 |
24.6 |
7.5 |
30.0 |
4.6 |
1.15 |
60.0 |
7.8 |
5.0 |
4.6 |
7.2 |
45.0 |
8.3 |
|
|
|
|
|
|
|
|
Fig. 1.2. Chemical structure of an inhibitor of the ubiquitin-conjugating enzyme E2B (Rad6B).
Interesting data on the cytotoxic activity of the triazine cycle are described in [10], where methods for obtaining derivatives of 2-(4-amino-6-(4-(2-chlorophenyl)piperazin-1- yl)-1,3,5- triazin-2-yl)acetonitrile 1.16 as a precursor in the synthesis of amino-4-{[4-(2- R'-phenyl)piperazino]-1,3,5-triazin-6-yl}acetonitrile 1.17. Target compounds were obtained by the interaction of N-carbamimidoyl-4-(2-chlorophenyl)piperazine-1- carboximidamide and ethylcyanoacetate in methanol solution at room temperature.
The intermediate 2-amino-4-(4-arylpiperazino)-1,3,5-triazine 1.16, coupled directly to the cyanovinyl pharmacophore, was used for the subsequent reaction with 5-nitro-2- furaldehyde in a triethylamine-catalysed Knoevenagel condensation reaction to give (E)- 2-(4-amino-6-(4-phenylpiperazin-1-yl)-1,3,5-triazin-2-yl)-3-(5-nitrofuran-2- yl)acrylonitrile 1.17 with a yield of 57 % (Scheme 1.5).

158
Scheme 1.5
Screening for cytotoxic activity was performed on 10 human tumour cell lines: cervical adenocarcinoma of the cervix (SISO), oesophageal squamous cell carcinoma (KYSE-520, KYSE-70, KYSE-510), large cell lung carcinoma (LCLC-103H), bladder carcinoma (RT-4), urinary tract carcinoma (RT-112 and RT-5637), pancreatic carcinoma (YAPC and DAN-G). Among the triazine derivatives, the highest cytotoxic activity was found for compound 1.17, which contains chlorine in the aryl ring (IC50 1.66 µM for RT-
4, 1.05 µM for RT-112, 0.92 µM for RT-5637, 0.92 µM for KYSE-70, 0.45 µM for KYSE510, 0.95 µM for KYSE-520, 0.72 µM for YAPC, 0.86 µM for DAN-G, 0.90 µM for SISO and 0.89 µM for LCLC-103H), which indicates that the introduction of halogen substituents into the structure of 1,3,5-triazines is promising.
Junaid et al. used microwave synthesis to obtain 6,N2-diaryl-1,3,5-triazine-2,4- diamines (Scheme 1.6) [11].
Рекомендовано к изучению сайтом МедУнивер - https://meduniver.com/

159
Scheme 1.6
The reaction between cyanoguanidine, aromatic aldehydes and arylamines in the presence of hydrochloric acid under the action of microwave irradiation led to the formation of Dimroth rearrangement products, which in turn undergo spontaneous dehydrogenation and aromatisation to give 6,N2-diaryl-1,3,5-triazine-2, 4-diamines (1.18 and 1.19) (Scheme 1.6).
The resulting compounds were tested for antiproliferative activity against the human prostate carcinoma cell line DU145 (C = 10 μM). Compounds 1.18 and 1.19 were shown to inhibit cell proliferation by more than 45 %.
1.2.Reactions of nucleophilic substitution of chlorine atoms in
2,4,6-trichloro-1,3,5-triazine derivatives
A method is considered, which consists in the successive nucleophilic substitution of chlorine atoms in the presence of a base (Scheme 1.7). The starting compound is 2,4,6- trichloro-1,3,5-triazine (cyanuric chloride, TXT).
Scheme 1.7
Despite the wide distribution of the presented synthesis method, it has significant drawbacks, mainly associated with the harsh conditions of the nucleophilic substitution of the third chlorine atom and low yields of the trisubstituted product [12,13].
160
1.2.1. Reactions of 2,4,6-trichloro-1,3,5-triazine with amines of various nature
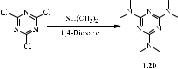
The first chlorine substitution product in TXT was N2,N2,N4,N4,N6,N6-hexamethyl- 1,3,5-triazine-2,4,6-triamine (altretamine), which was approved by the FDA under the brand name Hexalen® for the treatment of ovarian cancer [14,15]. The target trisubstituted TXT was synthesised by dimethylamination of TXT with an aqueous solution of 40 % dimethylamine and potassium hydroxide in 1,4-dioxane in one step to obtain altretamine (1.20) in high yield (Scheme 1.8) [16,17].
Scheme 1.8
In animals and humans, altretamine undergoes oxidative N-demethylation to form hydroxymethyl derivatives. It has been shown that it is hydroxymethylmelamines that are responsible for the cytotoxic and antitumour activity of altretamine [18]. Altretamine acts as an alkylating agent, but the detailed mechanism of its antitumour activity is unknown. Altretamine presumably damages tumour cells due to the formation of formaldehyde as a product of N-demethylation with the participation of cytochrome P450. When taken orally, altretamine is metabolised in the liver, forming mainly monoand didemethylated metabolites. In tumour cells, sequential reactions of demethylation and release of formaldehyde occur in situ. It has been shown that altretamine is able to covalently interact with guanine and cytosine residues that are part of DNA [19]. Altretamine after oral administration is well absorbed and undergoes rapid demethylation in the liver, causing a significant decrease in the concentration of the active substance in plasma. In subsequent studies in patients with recurrent ovarian cancer after Hexalen® monotherapy for a 14, 21 or 28-day cycle, 6 complete clinical responses and 2 partial responses were achieved with an overall response rate of 18%, with a duration of 2 to 36 months. In some patients, tumour regression was accompanied by an improvement in general condition [20].
The authors of [21] successfully performed the synthesis of thiourea derivatives, for example, compound 1.22 (without isolation of intermediate products) in yields up to 71 %.
Рекомендовано к изучению сайтом МедУнивер - https://meduniver.com/
