
- •Contents
- •Preface to the Third Edition
- •About the Authors
- •How to Use Herbal Medicines
- •Introduction
- •General References
- •Agnus Castus
- •Agrimony
- •Alfalfa
- •Aloe Vera
- •Aloes
- •Angelica
- •Aniseed
- •Apricot
- •Arnica
- •Artichoke
- •Asafoetida
- •Avens
- •Bayberry
- •Bilberry
- •Bloodroot
- •Blue Flag
- •Bogbean
- •Boldo
- •Boneset
- •Borage
- •Broom
- •Buchu
- •Burdock
- •Burnet
- •Butterbur
- •Calamus
- •Calendula
- •Capsicum
- •Cascara
- •Cassia
- •Cat’s Claw
- •Celandine, Greater
- •Celery
- •Centaury
- •Cereus
- •Chamomile, German
- •Chamomile, Roman
- •Chaparral
- •Cinnamon
- •Clivers
- •Clove
- •Cohosh, Black
- •Cohosh, Blue
- •Cola
- •Coltsfoot
- •Comfrey
- •Corn Silk
- •Couchgrass
- •Cowslip
- •Cranberry
- •Damiana
- •Dandelion
- •Devil’s Claw
- •Drosera
- •Echinacea
- •Elder
- •Elecampane
- •Ephedra
- •Eucalyptus
- •Euphorbia
- •Evening Primrose
- •Eyebright
- •False Unicorn
- •Fenugreek
- •Feverfew
- •Figwort
- •Frangula
- •Fucus
- •Fumitory
- •Garlic
- •Gentian
- •Ginger
- •Ginkgo
- •Ginseng, Eleutherococcus
- •Ginseng, Panax
- •Golden Seal
- •Gravel Root
- •Ground Ivy
- •Guaiacum
- •Hawthorn
- •Holy Thistle
- •Hops
- •Horehound, Black
- •Horehound, White
- •Horse-chestnut
- •Horseradish
- •Hydrangea
- •Hydrocotyle
- •Ispaghula
- •Jamaica Dogwood
- •Java Tea
- •Juniper
- •Kava
- •Lady’s Slipper
- •Lemon Verbena
- •Liferoot
- •Lime Flower
- •Liquorice
- •Lobelia
- •Marshmallow
- •Meadowsweet
- •Melissa
- •Milk Thistle
- •Mistletoe
- •Motherwort
- •Myrrh
- •Nettle
- •Parsley
- •Parsley Piert
- •Passionflower
- •Pennyroyal
- •Pilewort
- •Plantain
- •Pleurisy Root
- •Pokeroot
- •Poplar
- •Prickly Ash, Northern
- •Prickly Ash, Southern
- •Pulsatilla
- •Quassia
- •Queen’s Delight
- •Raspberry
- •Red Clover
- •Rhodiola
- •Rhubarb
- •Rosemary
- •Sage
- •Sarsaparilla
- •Sassafras
- •Saw Palmetto
- •Scullcap
- •Senega
- •Senna
- •Shepherd’s Purse
- •Skunk Cabbage
- •Slippery Elm
- •Squill
- •St John’s Wort
- •Stone Root
- •Tansy
- •Thyme
- •Uva-Ursi
- •Valerian
- •Vervain
- •Wild Carrot
- •Wild Lettuce
- •Willow
- •Witch Hazel
- •Yarrow
- •Yellow Dock
- •Yucca
- •1 Potential Drug–Herb Interactions
- •4 Preparations Directory
- •5 Suppliers Directory
- •Index
Thyme
Summary and Pharmaceutical Comment
Thyme is commonly used as a culinary herb and is characterised by its volatile oil. Documented pharmacological actions support some of the traditional medicinal uses, which have been principally attributed to the volatile oil and flavonoid constituents. However, robust clinical research assessing the efficacy and safety of thyme is limited. The oil is toxic and should not be ingested and only applied externally if diluted in a suitable carrier oil. It has been suggested that standardised thyme extracts based on the phenolic volatile components may not be appropriate because antispasmodic actions previously attributed to these compounds may be attributable to other constituents. Thyme should not be ingested during pregnancy and lactation in quantities greater than those found in foods.
Species (Family)
*Thymus vulgaris L.
†Thymus zygis L. (Labiatae/Lamiaceae)
Synonym(s)
Common Thyme, French Thyme, Garden Thyme, Rubbed Thyme *Thymus ilerdensis Gonz. Frag. ex Costa., T. webbianus Rouy, T. valentinus Rouy., T. aestivus Reut. ex Willk., T. welwitschii Boiss. subsp ilerdensis (Gonz. Frag. ex Costa) Nyman
†T. sabulicola Coss., T. sylvestris Hoffman & Link
Part(s) Used
Flowering top, leaf
Pharmacopoeial and Other Monographs
BHP 1996(G9)
BP 2007(G86)
Complete German Commission E(G3)
ESCOP 2003(G76)
Martindale 35th edition(G85)
Ph Eur 2007(G81)
WHO volume 1 1999(G63)
Legal Category (Licensed Products)
GSL(G37)
Constituents
The following is compiled from several sources, including General References G2, G22, G52 and G58.
Volatile oils 0.8–2.6%. Pharmacopoeial standard, not less than 1.2%.(G81, G86) Phenols as major components (20–80%) primarily
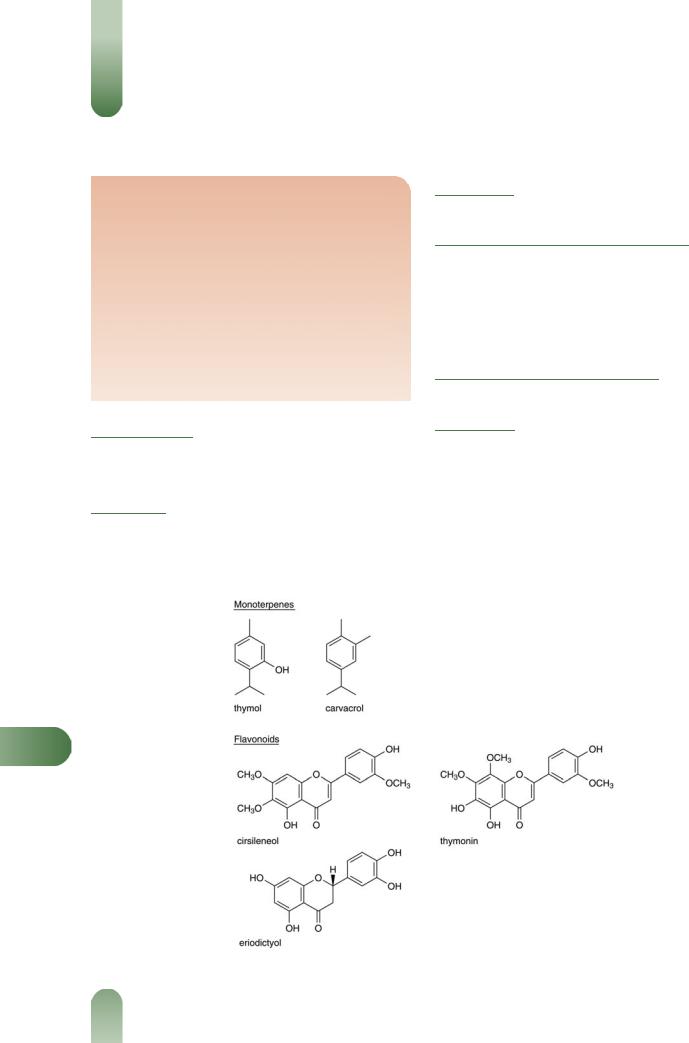
thymol and carvacrol; others include p-cymene and g-terpinene (monoterpenes), linalool, a-terpineol, and thujan-4-ol (alcohols); biphenyl compounds of monoterpene origin.(G52) A detailed analysis of the volatile oil components is given elsewhere.(G22)
Flavonoids Cirsilineol, 8-methoxycirsilineol, thymonin and eriodictyol.
T
Figure 1 Selected constituents of thyme.
574

Other constituents Caffeic acid, oleanolic acid, ursolic acid, rosmarinic acid, resins, saponins and tannins.
Food Use
Thyme is commonly used as a culinary herb, and thyme oil is used
in food flavouring. Previously, thyme has been listed as GRAS (Generally Recognised As Safe).(G65)
Herbal Use
Thyme is stated to possess carminative, antispasmodic, antitussive, expectorant, secretomotor, bactericidal, anthelmintic and astringent properties. Traditionally, it has been used for dyspepsia, chronic gastritis, asthma, diarrhoea in children, enuresis in
children, laryngitis, tonsillitis (as a gargle), and specifically for pertussis and bronchitis.(G2, G4, G7, G32, G43, G50, G52, G64) The Ger-
man Commission E approved internal use for treating symptoms of bronchitis, whooping cough and catarrh of the upper respiratory tract.(G3) Thyme is used in various combinations with anise oil, eucalyptus oil, fennel oil, fennel fruit, Iceland moss, lime flower, liquorice root, marshmallow root, primrose root and
star anise fruit for catarrh and diseases of the upper respiratory tract.(G3)
Dosage
Dosages for oral administration (adults) for traditional uses recommended in older and contemporary standard herbal and pharmaceutical reference texts are given below.
Dried herb 1–4 g as an infusion three times daily;(G7) 1–
2 g.(G3, G52)
Liquid Extract of Thyme (BPC 1949) 0.6–4.0 mL.
Elixir of Thyme (BPC 1949) 4–8 mL.
Tincture 2–6 mL (1 : 5 in 45% alcohol) three times daily,(G7) four
drops.(G3, G52)
Pharmacological Actions
In vitro and animal studies
Antitussive, expectorant and antispasmodic actions are considered to be the major pharmacological properties of thyme,(1) and have been associated with the volatile oils (e.g. thymol, carvacrol) and flavonoid constituents. Thyme oil has produced hypotensive and
Figure 2 Thyme (Thymus vulgaris).
Thyme 575
Figure 3 Thyme – dried drug substance (leaf).
respiratory stimulant effects in rabbits following oral or intramuscular administration, and in cats following intravenous injection;(G41) an increase in rhythmic heart contraction was also observed in rabbits.(G41)
In vitro antispasmodic activity of thyme and related Thymus |
|
||
species has been associated with the phenolic components of the |
|
||
volatile oil(2) and with the flavonoid constituents; their mode of |
|
||
action is thought to involve calcium-channel blockage.(1, 3, 4) The |
|
||
flavonoids thymonin, circilineol and 8-methoxycircilineol have |
|
||
potent spasmolytic activity in guineapig trachea preparations in |
|
||
vitro.(G52) |
|
||
Analgesic and antipyretic properties in mice have been reported |
|
||
for a thyme extract.(5) |
|
||
Thymol possesses anthelmintic (especially hookworms), anti- |
|
||
bacterial and antifungal properties.(G41) Thymol and thyme oil |
|
||
have antibacterial activity against certain organisms and thymol, |
|
||
carvacrol and thyme oil have antifungal activity against a range of |
|
||
organisms.(G50) |
|
||
Thyme oil inhibits prostaglandin synthesis; rosmarinic acid has |
|
||
anti-inflammatory activity, inhibiting complement in rats and |
|
||
some of the functions of polymorphonucleocytes.(G52) Rosmarinic |
|
||
acid reduced oedema produced by cobra venom factor in rats, and |
|
||
inhibited passive cutaneous anaphylaxis and impairment of in |
|
||
vivo activation of mouse macrophages by heat killed Corynebac- |
|
||
terium parvum.(G52) Activity may relate to complement inactiva- |
|
||
tion.(G50) |
|
||
Clinical studies |
|
||
Generally, well-designed clinical studies assessing the effects of |
T |
||
thyme are lacking. A randomised, double-blind, controlled trial |
|||
|
|||
involving 60 patients with productive cough compared syrup of |
|
||
thyme and bromhexine over a five-day period. Both groups were |
|
||
similar in self-reported symptom relief.(G50) |
|
||
Side-effects, Toxicity |
|
|
|
Clinical data |
|
||
There is a lack of clinical safety and toxicity data for thyme and |
|
||
further investigation of these aspects is required. |
|
||
Thyme oil is a dermal and mucous membrane irritant.(G58) |
|
||
Toxic symptoms documented for thymol include nausea, vomit- |
|
||
ing, gastric pain, headache, dizziness, convulsions, coma, and |
|
||
cardiac and respiratory arrest.(G22) Thymol is present in some |
|
||
toothpaste preparations, and has been reported to cause cheilitis |
|
||

576 Thyme
and glossitis. Hyperaemia and severe inflammation have been described for thyme oil used in bath preparations.(G51)
Preclinical data
A concentrated extract of thyme decreased locomotor activity and caused a slight slowing down of respiration in mice following oral
administration of doses of 0.5–3.0 g/kg, equivalent to 4.3–26.0 g dried plant material.(G52) In rats, oral LD50 values stated for thyme oil include 2.84 g/kg(G52) and 4.7 g/kg in rats, and >5 g/kg
following dermal administration.(6) In mice, oral administration of a concentrated ethanol extract of herb in subacute toxicity tests resulted in increased weights of liver and testes. Also in mice, a dose of 0.9 g daily for three months resulted in mortality rates of 30% and 10% in males and females, respectively. Thyme oil had no mutagenic or DNA-damaging activity in either the Ames test or Bacillus subtilis rec-assay.(G52)
Contra-indications, Warnings
Thyme oil is toxic and should be used with considerable caution. It should not be taken internally and only applied externally if diluted in a suitable carrier oil.
Drug interactions None documented. However, the potential for preparations of thyme to interact with other medicines administered concurrently, particularly those with similar or opposing effects, should be considered.
Pregnancy and lactation There are no known problems with the use of thyme during pregnancy and lactation, provided that doses do not greatly exceed the amounts used in foods. Traditionally, thyme is reputed to affect the menstrual cycle and, therefore, large amounts should not be ingested.
Preparations
Proprietary single-ingredient preparations
Austria: Scottopect. Czech Republic: Bronchicum HustenPastillen. Germany: Anastil; Aspecton; Biotuss; Bronchicum Pastillen; Bronchipret; Gelobronchial; Hustagil ThymianHustensaft; Hustagil Thymiantropfen; Husties; Isephca S; Makatussin Saft; Melrosum Hustensirup; Mirfusot; Nimopect; Pertussin; Sanopinwern; Soledum Hustensaft; Soledum Hustentropfen; Tetesept Erkaltungs; Thymipin N; Thymiverlan; Tussamag Hustensaft N; Tussamag Hustentropfen N; Tussiflorin Thymian. Israel: Thymi Syrup. Russia: Bronchicum
THusten (Бронхикум Пастилки от Кашля); Tussamag (Тусса-
маг). Switzerland: Thymusin N.
Proprietary multi-ingredient preparations
Argentina: Expectosan Hierbas y Miel. Australia: Broncafect; Cough Relief; Euphorbia Complex. Austria: Bronchipret; Bronchithym; Bronchostop; Brustund Hustentee St Severin; Codelum; Eicebaer; Expectal-Tropfen; Krauter Hustensaft; Luuf-Hustentee; Paracodin; Pilka Forte; Pilka; Pneumopan; Pneumopect; Scottopect; Thymoval; Tussamag; Tussimont. Belgium: Colimax. Canada: Herbal Throat; Original Herb Cough Drops; Swiss Herb Cough Drops. Chile: Phyto Corrective Gel; Rhus Opodeldoc. Czech Republic: Biotussil; Bronchialtee N; Bronchicum Elixir; Bronchicum Hustensirup; Bronchicum Sekret-Loser; Bronchicum Tropfen; Erkaltungstee; Nontusyl; Perospir; Pleumolysin; Pulmoran; Stomatosan; Thymomel; Urcyston Planta. Finland: Katapekt. France: Depuratum; Saugella; Tussidoron. Germany: Bronchicum Elixir S; Bronchicum; Bronchipret; Bronchipret; Brustund Hustentee; Cefadrin; Cito-Guakalin; Drosithym-N; Em-med- ical; Ephepect-Pastillen N; Equisil N; Expectysat N; Harzer Hustenloser; Kinder Em-eukal Hustensaft; Lomal; Makatussin Tropfen; Melissengeist; Muc-Sabona; Phytobronchin; Pulmotin; Sinuforton; Sinuforton; TUSSinfant N. Israel: Pilka. Italy: Broncosedina; Immumil; Piodermina; Saugella Attiva; Saugella Fitothym; Saugella Poligyn 7; Tussol. Netherlands: Balsoclase Compositum; Bronchicum. Russia: Bronchicum (Бронхикум); Bronchicum Husten (Бронхикум Сироп от Кашля); Maraslavin (Мараславин); Stoptussin-Fito (Стоптуссин-фито). South Africa: Cough Elixir. Spain: Natusor Asmaten; Natusor Farinol; Natusor Gripotul; Natusor Infenol; Natusor Renal; Natusor Sinulan; Pilka; Wobenzimal; Wobenzimal. Switzerland: DemoPectol; DemoPectol; Makaphyt Gouttes antitussives; Makaphyt Sirop; Nican; Pectoral N; Strath Gouttes contre la toux; Tisane pectorale et antitussive; Tisane pectorale pour les enfants. Thailand: Solvopret; Solvopret TP. UK: Herb and Honey Cough Elixir. Venezuela: Codebromil; Mixagel.
References
1Van Den Broucke CO. The therapeutic value of Thymus species. Fitoterapia 1983; 4: 171–174.
2Van Den Broucke CO, Lernli JA. Pharmacological and chemical investigation of thyme liquid extracts. Planta Med 1981; 41: 129–135.
3 Cruz T et al. The spasmolytic activity of the essential oil of Thymus baeticus Boiss in rats. Phytother Res 1989; 3: 106–108.
4Blázquez MA et al. Effects of Thymus species extracts on rat duodenum isolated smooth muscle contraction. Phytother Res 1989; 3:
41–42.
5Mohsin A et al. Analgesic, antipyretic activity and phytochemical screening of some plants used in traditional Arab system of medicine.
Fitoterapia 1989; 60: 174.
6Opdyke DLJ. Thyme oil, red. Food Cosmet Toxicol 1974; 12: 1003– 1004.

Uva-Ursi
Summary and Pharmaceutical Comment
The chemistry of uva-ursi is well documented with hydroquinone derivatives, especially arbutin, identified as the major active constituents. Documented pharmacological actions justify the herbal use of uva-ursi as a urinary antiseptic. However, clinical information is lacking and further studies are required to determine the true usefulness of uva-ursi in the treatment of urinary tract infections. Although hydroquinone has been reported to be toxic in large amounts, concentrations provided by the ingestion of therapeutic doses of uva-ursi are not thought to represent a risk to human health. Excessive use of uva-ursi and use during pregnancy and lactation should be avoided.
Species (Family)
Arctostaphylos uva-ursi (L.) Spreng. (Ericaceae)
Synonym(s)
Bearberry
Part(s) Used
Leaf
Pharmacopoeial and Other Monographs
BHC 1992(G6)
BHP 1996(G9)
BP 2007(G84)
Complete German Commission E(G3)
ESCOP 2003(G76)
Martindale 35th edition(G85)
Ph Eur 2007(G81)
Legal Category (Licensed Products)
GSL(G37)
Constituents
The following is compiled from several sources, including General References G2 and G6.
Flavonoids Flavonols (e.g. myricetin, quercetin) and their glycosides including hyperin, isoquercitrin, myricitrin and quercitrin.
Iridoids Asperuloside (disputed), monotropein.(1)
Quinones Total content at least 6%, mainly arbutin (5–15%) and methyl-arbutin (glycosides), with lesser amounts of piceoside(2) (a glycoside), free hydroquinone and free p-methoxyphenol.(3)
Tannins 6–7% (range 6–40%). Hydrolysable-type (e.g. corilagin pyranoside); ellagic and gallic acids (usually associated with hydrolysable tannins).
Figure 1 Selected constituents of uva-ursi.
Terpenoids a-Amyrin, a-amyrin acetate, b-amyrin, lupeol, uvaol, ursolic acid, and a mixture of monoand di-ketonic a- amyrin derivatives.(4, 5)
Other constituents Acids (malic, quinic), allantoin, resin (e.g. ursone), volatile oil (trace) and wax.
Other plant parts The root is reported to contain unedoside (iridoid glucoside).(6)
Food Use
Uva-ursi is not used in foods.
Herbal Use
Uva-ursi is stated to possess diuretic, urinary antiseptic, and astringent properties. Traditionally, it has been used for cystitis, urethritis, dysuria, pyelitis, lithuria, and specifically for acute
catarrhal cystitis with dysuria and highly acidic urine.(G2, G6, G7,
G8, G64)
Dosage
Dosages for oral administration (adults) for traditional uses recommended in older and contemporary standard herbal and pharmaceutical reference texts are given below.
U
Figure 2 Uva-ursi (Arctostaphylos uva-ursi).
577

578 Uva-Ursi
Figure 3 Uva-ursi – dried drug substance (leaf).
Dried leaves 1.5–4.0 g as an infusion three times daily.(G6, G7)
Liquid extract 1.5–4.0 mL (1 : 1 in 25% alcohol) three times
daily.(G6, G7)
Concentrated Infusion of Bearberry (BPC 1934) 2–4 mL.
Fresh Infusion of Bearberry (BPC 1934) 15–30 mL.
Pharmacological Actions
In vitro and animal studies
Uva-ursi has exhibited antimicrobial activity towards a variety of organisms including Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Mycobacterium smegmatis, Shigella sonnei and
Shigella flexneri.(7) The antimicrobial activity of arbutin towards bacteria implicated in producing urinary tract infections, has been found to be directly dependent on the b-glucosidase activity of the infective organism.(8) Highest enzymatic activity was shown by
Enterobacter, Klebsiella and Streptococcus genera, and lowest by
Escherichia coli.(8) The minimum inhibitory concentration for arbutin is reported to be 0.4–0.8% depending on the microorganism.(8) Aqueous and methanolic extracts have demonstrated molluscicidal activity against Biomphalaria glabrata, at a concentration of 50 ppm.(9) The activity was attributed to the tannin constituents (condensed and hydrolysable).
Anti-inflammatory activity (rat paw oedema tests) has been documented for uva-ursi against a variety of chemical inducers such as carrageenan, histamine and prostaglandins.(10)
Uva-ursi failed to exhibit any in vitro uterotonic action when tested on rabbit and guinea-pig uteri.(11)
Hydroquinone has been reported to show a concentration-
Udependent cytotoxic activity on cultured rat hepatoma cells (HTC line); arbutin was not found to inhibit growth of the HTC cells.(12) It was stated that hydroquinone appeared to have greater cytotoxic activity towards rat hepatoma cells than agents like azauridin or colchicine, but less than valtrate from valerian
(Valeriana officinalis). The cytoxicity of hydroquinone has also been tested on L1210, CA-755 and S-180 tumour systems.(12)
Clinical studies
Clinical research assessing the effects of uva-ursi is limited and rigorous randomised clinical trials are required.
A herbal preparation, whose ingredients included uva-ursi,
hops and peppermint, has been used to treat patients suffering from compulsive strangury, enuresis and painful micturition.(13)
Of 915 patients treated for six weeks, success was reported in about 70%. The design of this study, however, does not allow the observed effects to be attributed to uva-ursi.
The antiseptic and diuretic properties claimed for uva-ursi can be attributed to the hydroquinone derivatives, especially arbutin. The latter is absorbed from the gastrointestinal tract virtually unchanged and during renal excretion is hydrolysed to yield the active principle, hydroquinone, which exerts an antiseptic and astringent action on the urinary mucous membranes.(14, 15) The
crude extract is reported to be more effective than isolated arbutin as an astringent and antiseptic.(G48) This may be due to the other
hydroquinone derivatives, in addition to arbutin, that are present in the crude extract and which will also yield hydroquinone. Furthermore, it has been stated that the presence of gallic acid in the crude extract may prevent b-glucosidase cleavage of arbutin in the gastrointestinal tract before absorption, thereby increasing the amount of hydroquinone released during renal excretion.(G48)
Side-effects, Toxicity
Clinical data
There is a lack of clinical safety and toxicity data for uva-ursi and further investigation of these aspects is required.
Hydroquinone is reported to be toxic if ingested in large quantities: 1 g (equivalent to 6–20 g plant material) has caused tinnitus, nausea and vomiting, sense of suffocation, shortness of breath, cyanosis, convulsions, delirium and collapse.(G48) A dose
of 5 g (equivalent to 30–100 g of plant material) has proved fatal.(G48) In view of the high tannin content, prolonged use of uva-ursi may cause chronic liver impairment.(G41)
Preclinical data
Cytotoxic activity has been documented for hydroquinone (see Pharmacological Actions, In vitro and animal studies).
Uva-ursi herb can sometimes be adulterated with box leaves (Buxus sempervirens), which contain toxic steroidal alkaloids.
However, no cases of poisoning as a result of such adulteration have been reported.(G33)
Contra-indications, Warnings
Uva-ursi requires an alkaline urine for it to be effective as a urinary antiseptic; an alkaline reaction is needed to yield hydroquinone from the inactive esters such as arbutin.(14) Patients have been advised to avoid eating highly acidic foods, such as acidic fruits and their juices.(14) The presence of hydroquinone may impart a greenish-brown colour to the urine, which darkens following exposure to air due to oxidation of hydroquinone.
Excessive use of uva-ursi should be avoided in view of the high tannin content and potential toxicity of hydroquinone.
Prolonged use of uva-ursi to treat a urinary tract infection is not advisable. Patients in whom symptoms persist for longer than 48 hours should consult their doctor.
Drug interactions None documented. However, the potential for preparations of uva-ursi to interact with other medicines administered concurrently, particularly those with similar or opposing effects, should be considered.
Pregnancy and lactation Large doses of uva-ursi are reported to be oxytocic,(G22) although in vitro studies have reported a lack of
utero-activity. In view of the potential toxicity of hydroquinone, the use of uva-ursi during pregnancy and lactation should be avoided.

Preparations
Proprietary single-ingredient preparations
Czech Republic: List Medvedice Lecive; Medvedice. Germany: Cystinol Akut; Uvalysat. Mexico: Uvavid.
Proprietary multi-ingredient preparations
Argentina: Ajolip; KLB6 Fruit Diet. Australia: Althaea Complex; Bioglan Cranbiotic Super; Cranberry Complex; Cranberry Complex; De Witts New Pills; Extralife Fluid-Care; Extralife PMS-Care; Extralife Uri-Care; Fluid Loss; Herbal Diuretic Formula; Medinat PMT-Eze; Profluid; Protemp; Urinase; Uva-Ursi Complex; Uva-Ursi Plus. Austria: Uropurat. Brazil: Composto Anticelulitico; Emagrevit; Lisian; Pilulas De Witt's. Canada: Herbal Diuretic. Czech Republic: Blasenund Nierentee; Species Urologicae Planta; Urcyston Planta; Urologicka Cajova Smes. France: Mediflor Tisane Antirhumatismale No 2; Mediflor Tisane No 4 Diuretique; Uromil; Urophytum. Germany: Arctuvan; Cystinol; Harntee STADA. Israel: Jungborn. Mexico: Noxivid. New Zealand: De Witts Pills. Portugal: Asic; Rilastil Dermo Solar. Russia: Herbion Urological Drops (Гербион Урологические Капли). Spain: Genurat; Urisor. Switzerland: Demonatur Dragees pour les reins et la vessie; Dragees S pour les reins et la vessie; Strath Gouttes pour les reins et la vessie; Tisane pour les reins et la vessie; Urinex. UK: Antitis; Aqua Ban Herbal; Backache Relief; Backache; Backache; Buchu Backache Compound Tablets; Cascade; De Witt's K & B Pills; Diuretabs; Fenneherb Cystaid; Fenneherb Prementaid; HealthAid Boldo-Plus; HRI Water Balance; KasBah; Modern Herbals Water Retention; Napiers Uva Ursi Tea; Prementaid; Sciargo; Sciatica Tablets; Tabritis; Tabritis Tablets; Uvacin; Watershed. USA: CitriMax Plus.
Uva-Ursi 579
References
1Jahodár L et al. Investigation of iridoid substances in Arctostaphylos uva-ursi. Pharmazie 1978; 33: 536–537.
2Karikas GA et al. Isolation of piceoside from Arctostaphylos uva-ursi. Planta Med 1987; 53: 307–308.
3Jahodár L, Leifertová I. The evaluation of p-methoxyphenol in the leaves of Arctostaphylos uva-ursi. Pharmazie 1979; 34: 188–189.
4 Droliac A. Triterpenes of Arctostaphylos uva-ursi Spreng. Plant Méd Phytothér 1980; 14: 155–158.
5Malterud KE. The non-polar components of Arctostaphylos uva-ursi leaves. Medd Nor Farm Selsk 1980; 42: 15–20.
6 Jahodár L et al. Unedoside in Arctostaphylos uva-ursi roots. Pharmazie 1981; 36: 294–296.
7Moskalenko SA. Preliminary screening of far-Eastern ethnomedicinal plants for antibacterial activity. J Ethnopharmacol 1986; 15: 231–259.
8 Jahodár L et al. Antimicrobial action of arbutin and the extract from the leaves of Arctostaphylos uva-ursi in vitro. Ceskoslov Farm 1985; 34: 174–178.
9 Schaufelberger D, Hostettmann K. On the molluscicidal activity of tannin containing plants. Planta Med 1983; 48: 105–107.
10Shipochliev T, Fournadjiev G. Spectrum of the antiinflammatory effect of Arctostaphylos uva ursi and Achillea millefolium, L. Probl Vutr Med 1984; 12: 99–107.
11Shipochliev T. Extracts from a group of medicinal plants enhancing the uterine tonus. Vet Med Nauki 1981; 18: 94–98.
12Assaf MH et al. Preliminary study of the phenolic glycosides from Origanum majorana; quantitative estimation of arbutin; cytotoxic activity of hydroquinone. Planta Med 1987; 53: 343–345.
13Lenau H et al. Wirksamkeit und Verträglichkeit von Cysto Fink bei Patienten mit Reizblase und/oder Harninkontinenz. Therapiewoche 1984; 34: 6054–6059.
14Frohne D. Untersuchungen zur Frage der Harndesinfizierenden Wirkungen von Bärentraubenblatt-Extrakten. Planta Med 1970; 18: 23–25.
15Natural drugs with glycosides. In: Stahl E, ed. Drug Analysis in Chromatography and Microscopy. Ann Arbor: Ann Arbor Scientific Publishers, 1973: 97.
U
