
- •Contents
- •Preface to the Third Edition
- •About the Authors
- •How to Use Herbal Medicines
- •Introduction
- •General References
- •Agnus Castus
- •Agrimony
- •Alfalfa
- •Aloe Vera
- •Aloes
- •Angelica
- •Aniseed
- •Apricot
- •Arnica
- •Artichoke
- •Asafoetida
- •Avens
- •Bayberry
- •Bilberry
- •Bloodroot
- •Blue Flag
- •Bogbean
- •Boldo
- •Boneset
- •Borage
- •Broom
- •Buchu
- •Burdock
- •Burnet
- •Butterbur
- •Calamus
- •Calendula
- •Capsicum
- •Cascara
- •Cassia
- •Cat’s Claw
- •Celandine, Greater
- •Celery
- •Centaury
- •Cereus
- •Chamomile, German
- •Chamomile, Roman
- •Chaparral
- •Cinnamon
- •Clivers
- •Clove
- •Cohosh, Black
- •Cohosh, Blue
- •Cola
- •Coltsfoot
- •Comfrey
- •Corn Silk
- •Couchgrass
- •Cowslip
- •Cranberry
- •Damiana
- •Dandelion
- •Devil’s Claw
- •Drosera
- •Echinacea
- •Elder
- •Elecampane
- •Ephedra
- •Eucalyptus
- •Euphorbia
- •Evening Primrose
- •Eyebright
- •False Unicorn
- •Fenugreek
- •Feverfew
- •Figwort
- •Frangula
- •Fucus
- •Fumitory
- •Garlic
- •Gentian
- •Ginger
- •Ginkgo
- •Ginseng, Eleutherococcus
- •Ginseng, Panax
- •Golden Seal
- •Gravel Root
- •Ground Ivy
- •Guaiacum
- •Hawthorn
- •Holy Thistle
- •Hops
- •Horehound, Black
- •Horehound, White
- •Horse-chestnut
- •Horseradish
- •Hydrangea
- •Hydrocotyle
- •Ispaghula
- •Jamaica Dogwood
- •Java Tea
- •Juniper
- •Kava
- •Lady’s Slipper
- •Lemon Verbena
- •Liferoot
- •Lime Flower
- •Liquorice
- •Lobelia
- •Marshmallow
- •Meadowsweet
- •Melissa
- •Milk Thistle
- •Mistletoe
- •Motherwort
- •Myrrh
- •Nettle
- •Parsley
- •Parsley Piert
- •Passionflower
- •Pennyroyal
- •Pilewort
- •Plantain
- •Pleurisy Root
- •Pokeroot
- •Poplar
- •Prickly Ash, Northern
- •Prickly Ash, Southern
- •Pulsatilla
- •Quassia
- •Queen’s Delight
- •Raspberry
- •Red Clover
- •Rhodiola
- •Rhubarb
- •Rosemary
- •Sage
- •Sarsaparilla
- •Sassafras
- •Saw Palmetto
- •Scullcap
- •Senega
- •Senna
- •Shepherd’s Purse
- •Skunk Cabbage
- •Slippery Elm
- •Squill
- •St John’s Wort
- •Stone Root
- •Tansy
- •Thyme
- •Uva-Ursi
- •Valerian
- •Vervain
- •Wild Carrot
- •Wild Lettuce
- •Willow
- •Witch Hazel
- •Yarrow
- •Yellow Dock
- •Yucca
- •1 Potential Drug–Herb Interactions
- •4 Preparations Directory
- •5 Suppliers Directory
- •Index
Horehound, Black
Summary and Pharmaceutical Comment
Limited information is available on the chemistry of black horehound. A small number of studies have investigated pharmacological properties of isolated constituents and this information goes some way towards supporting some of the traditional uses, although there is a lack of basic experimental and clinical research into the effects of preparations of black horehound. In view of the lack of data on pharmacological effects, efficacy and safety, the appropriateness of medicinal use of black horehound should be considered. Excessive use,
Hduring pregnancy and breastfeeding should be avoided. The
potential for black horehound to interact with other medicines should be considered.at least, should be avoided. The use of black horehound
Species (Family)
Ballota nigra L. (Labiatae)
Synonym(s)
Ballota, Ballota foetida Lam.
Part(s) Used
Herb
Pharmacopoeial and Other Monographs
BHP 1996(G9)
BP 2007(G84)
Legal Category (Licensed Products)
Black horehound is not included in the GSL.(G37)
Constituents
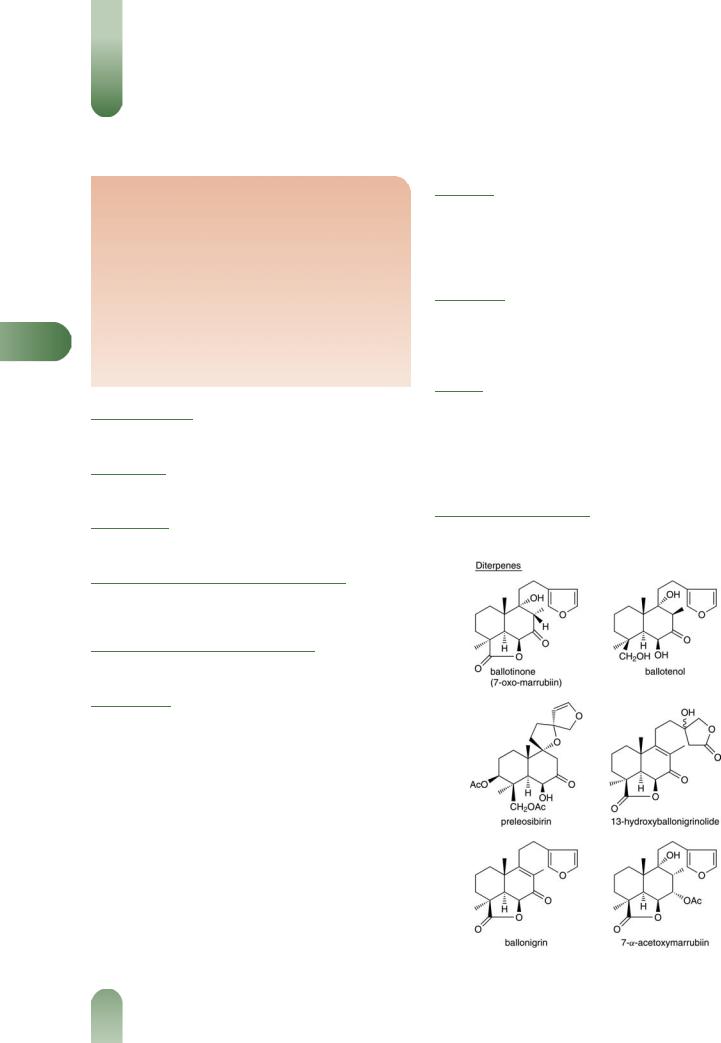
Diterpenes Ballotinone (7-oxomarrubiin),(1) ballotenol,(2) preleosibirin,(3) 13-hydroxyballonigrinolide, ballonigrin,(4, 5) and 7-a- acetoxymarrubiin.(6)
Flavonoids Lactoylated flavonoids 7-O-(2S-b-D-glucopyranosyl- oxypropanoyl)-luteolin and 7-O-(2S-hydroxypropanoyl)-luteolin (luteolin-7-lactate),(7) apigenin-7-glucoside, vicenin-2.(8)
Phenylpropanoids Verbascoside (acteoside), forsythoside B, arenarioside, ballotetroside, alyssonoside, lavandulifoliside, angoroside, caffeoylmalic acid.(5, 7, 9–12)
Essential oil B. nigra ssp. foetida 0.02%, major components b- caryophyllene and germacrene D.(13)
Related species Diterpenes from B. africana, B. andreuzziana, B. aucheri, B. hispanica, B. inaequidens, B. pseudodictamnus, B. lanata, B. rupestris and B. saxatilis, and flavonoids from B. acetabulosa, B. glandulissima, B. hirsuta, B. inequidens, B. pseudodictamnus and B. saxatilis.(14)
Food Use
Black horehound is listed by the Council of Europe as a natural source of food flavouring (category N3). This category indicates that black horehound can be added to foodstuffs in the traditionally accepted manner, although insufficient information is available for an adequate assessment of potential toxicity.(G16)
Herbal Use
Black horehound is stated to possess anti-emetic, sedative and mild astringent properties. Traditionally, it has been used for
nausea, vomiting, nervous dyspepsia, and specifically for vomiting of central origin.(G7, G64)
Dosage
Dosages for oral administration (adults) for traditional uses recommended in standard herbal reference texts are given below.
Dried herb 2–4 g as an infusion three times daily.(G7)
Liquid extract 1–3 mL (1 : 1 in 25% alcohol) three times daily.(G7)
Tincture 1–2 mL (1 : 10 in 45% alcohol) three times daily.(G7)
Pharmacological Actions
There is a lack of basic experimental and clinical research into the effects of preparations of black horehound, although a small
Figure 1 Selected constituents of black horehound.
358

number of studies has investigated pharmacological properties of isolated constituents.
In-vitro and animal studies
Certain phenylpropanoid derivatives isolated from the aerial parts of black horehound show pharmacological properties in vitro, including antibacterial, antioxidant and neurosedative activities. The phenylpropanoid glycosides arenarioside, forsythoside B and verbascoside, and the non-glycosidic phenylpropanoid caffeoylmalic acid bind to benzodiazepine, dopaminergic and morphinic receptors in vitro.(8) In radiolabelled ligand receptor binding assays using rat brain preparations, IC50 (concentrations required for 50% inhibition of radioligand binding) values for these compounds for each of the three receptor types were all < 5.0 mg/ mL. By contrast, ballotetroside did not show any affinity for binding to the receptors studied.
Arenarioside, forsythoside B and verbascoside inhibited the growth of one strain of the Gram-negative bacterium Proteus mirabilis and two strains of the Gram-positive bacterium Staphylococcus aureus, including a methicillin-resistant strain of S. aureus (minimum inhibitory concentration to prevent visible growth = 128 mg/mL).(12) Arenarioside also inhibited the growth of one strain of P. mirabilis when incubated at a concentration of 64 mg/mL. The compounds tested did not show any activity against Enterococcus faecalis, and the Gram-negative bacteria
Pseudomonas aeruginosa, Escherichia coli, Enterobacter aerogenes, Klebsiella pneumoniae and K. oxytoca.(12)
Horehound, Black |
359 |
H
Figure 3 Black horehound (Ballota nigra).
Arenarioside, forsythoside B and verbascoside, as well as ballotetroside, another phenylpropanoid glycoside isolated from the aerial parts of black horehound, have antioxidant properties in vitro.(11) These compounds inhibited copper ion-induced oxidation of low-density lipoproteins (LDL) with ED50 (efficacious concentration) values of 1.8, 1.0, 1.0 and 7.5 mmol/L, respectively. (LDL oxidation may be involved in the pathogenesis of atherosclerosis.) By comparison, quercetin, a known inhibitor of copper ion-induced LDL oxidation had an ED50 value of 2.3 mmol/ L. Further experiments showed that whereas quercetin chelated copper ions, the phenylpropanoid glycosides tested did not, indicating that their inhibition of copper ion-induced LDL oxidation is independent of any capacity for copper ion chelation(11)
In further experiments involving cell-free systems, arenarioside, ballotetroside, forsythoside B and verbascoside, as well as the nonglycosidic phenylpropanoid caffeoylmalic acid, demonstrated a concentration-dependent scavenging capacity towards several reactive oxygen species (ROS).(8) For each of the compounds, the
Figure 2 Selected constituents of black horehound. |
Figure 4 Black horehound – dried drug substance (herb). |

360 Horehound, Black
greatest ROS scavenging activity was seen for hydrogen peroxide and hypochlorous acid (concentrations required for 50% inhibition were 4 5.0 mg/L for all compounds, except ballotetroside, < 12 mg/L). Concentration-dependent inhibitions of ROS production by phorbol ester-stimulated PMNs were also seen in cellular systems following incubation with forsythoside B, verbascoside and caffeoylmalic acid, whereas arenarioside and ballotetroside showed little or no activity.(8)
Clinical studies
There is a lack of clinical research assessing the effects of black horehound and rigorous randomised controlled clinical trials are required.
Side-effects, Toxicity
None documented. However, there is a lack of clinical safety and
Htoxicity data for black horehound and further investigation of these aspects is required.
In in vitro experiments, the release of lactate dehydrogenase from polymorphonuclear neutrophils (PMN), an indicator of toxicity, was maintained below 20% following incubation with arenarioside, ballotetroside, forsythoside B, verbascoside and
caffeoylmalic acid at concentrations ranging from 0.1 to 200 mg/ L.(8) A report of the study states that this effect was not significant (p < 0.01), although it is not clear what was the control.
Contra-indications, Warnings
None documented.
Drug interactions None documented. However, the potential for preparations of black horehound to interact with other medicines administered concurrently, particularly those with similar or opposing effects, should be considered.
Pregnancy and lactation Black horehound is reputed to affect
the menstrual cycle although the scientific basis for this statement is not clear.(G30) In view of the lack of phytochemical,
pharmacological and toxicity data, the use of black horehound during pregnancy and breastfeeding should be avoided.
References
1Savona G et al. Structure of ballotinone, a diterpenoid from Ballota nigra. J Chem Soc Perkin Trans 1 1976; 1607–1609.
2Savona G et al. The structure of ballotenol, a new diterpenoid from
Ballota nigra. J Chem Soc Perkin Trans 1 1977; 497–499.
3Bruno M et al. Preleosibirin, a prefuranic labdane diterpene from
Ballota nigra subsp. foetida. Phytochemistry 1986; 25: 538–539.
4 Seidel V et al. Isolation from Ballota nigra L. of 13hydroxyballonigrinolide, a diterpene useful for the standardization of the drug. J Pharm Belg 1996; 51: 72–73.
5 Seidel V et al. Diterpène esters hétérosidiques phénylpropanoïques de
Ballota nigra L. Ann Pharm Franc 1998; 56: 31–35.
6 Savona G et al. Structures of three new diterpenoids from Ballota species. J Chem Soc Perkin Trans 1 1997; 5: 322–324.
7Bertrand MO et al. Two major flavonoids from Ballota nigra. Biochem Syst Ecol 2000; 28: 1031–1033.
8 Daels-Rakotoarison DA et al. Neurosedative and antioxidant activitites of phenylpropanoids from Ballota nigra. Arzneim Forsch 2000; 50: 16–23.
9 Seidel V et al. Phenylpropanoid glycosides from Ballota nigra. Planta Med 1996; 62: 186–187.
10Seidel V et al. A phenylpropanoid glycoside from Ballota nigra. Phytochemistry 1997; 44(4): 691–693.
11Seidel V, et al. Phenylpropanoids from Ballota nigra L. inhibit in vitro LDL peroxidation. Phytotherapy Res 2000; 14: 93–98.
12Didry N et al. Isolation and antibacterial activity of phenylpropanoid derivatives from Ballota nigra. J Ethnopharmacol 1999; 67: 197–202.
13Bader A et al. Composition of the essential oil of Ballota undulata, B. nigra ssp. foetida and B. saxatilis. Flavour Frag Journal 2003; 18: 502– 504.
14Sever Yilmiz B, Saltan Çitogˇ lu G.[Chemical constituents of Ballota L. species]. Ankara Ecz Fak Derg 2003; 32: 37–53 [Turkish].
Horehound, White
Summary and Pharmaceutical Comment
The chemistry of white horehound is well documented. Limited pharmacological information is available, although expectorant properties have been reported which support some of the herbal uses. There is a lack of robust clinical research assessing the efficacy and safety of white horehound. In view of the lack of toxicity data and suggested cardioactive properties, excessive use of white horehound and use during pregnancy and lactation should be avoided.
Species (Family)
Marrubium vulgare L. (Labiatae)
Synonym(s)
Common Hoarhound, Hoarhound, Horehound, Marrubium
Part(s) Used
Flower, leaf
Pharmacopoeial and Other Monographs
BHC 1992(G6)
BHP 1996(G9)
BP 2007(G84)
Complete German Commission E(G3)
Martindale 35th edition(G85)
Legal Category (Licensed Products)
GSL (G37)
Constituents
The following is compiled from several sources, including General References G2, G6 and G62.
Alkaloids Pyrrolidine-type. Betonicine 0.3%, the cis-isomer turicine.
Flavonoids Apigenin, luteolin, quercetin, and their glycosides.(1)
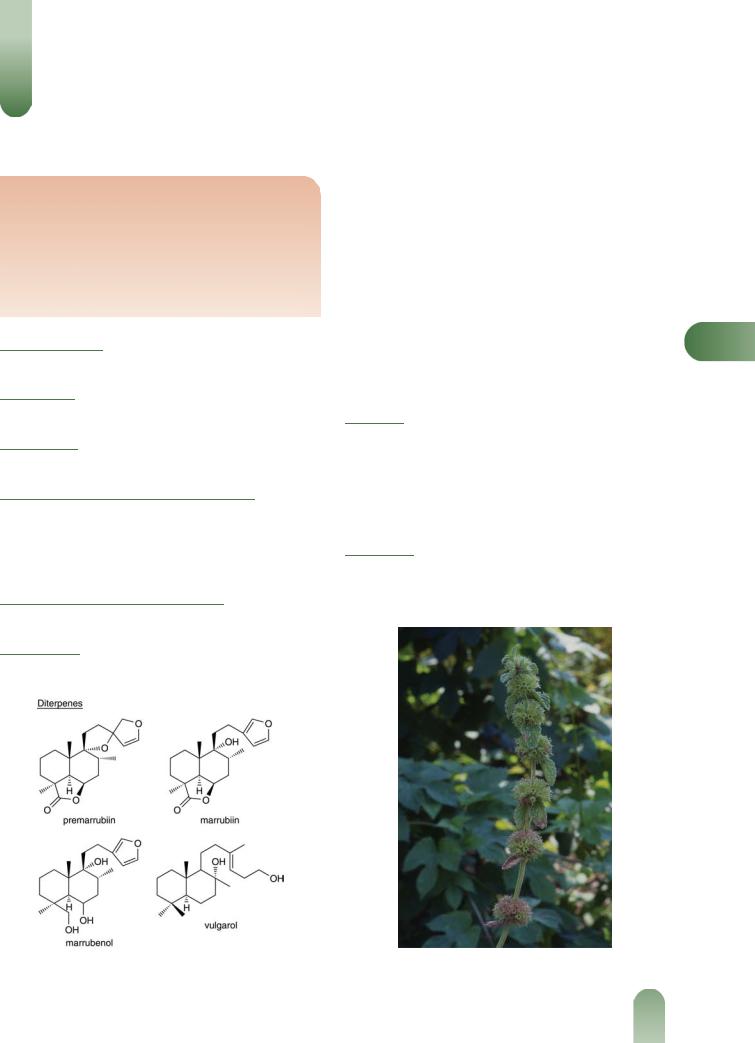
Terpenoids Diterpenes including marrubiin 0.3–1.0%, a lactone, as the main component with lesser amounts of various alcohols (e.g. marrubenol, marrubiol, peregrinol and vulgarol). Marrubiin has also been stated to be an artefact formed from a precursor, premarrubiin, during extraction.(2)
Volatile oils Trace. Bisabolol, camphene, p-cymene, limonene, b-
pinene, sabinene and others,(2) |
a sesquiterpene (unspecified). |
H |
|
Other constituents Choline, saponin (unspecified), b-sitosterol (a phytosterol), waxes (C26-C34 alkanes).
Food Use
White horehound is listed by the Council of Europe as a natural source of food flavouring (category N2). This category indicates that white horehound can be added to foodstuffs in small quantities, with a possible limitation of an active principle (as yet
unspecified) in the final product.(G16) Previously, white horehound has been listed as GRAS (Generally Recognised As Safe).(G65)
Herbal Use
White horehound is stated to possess expectorant and antispasmodic properties. Traditionally, it has been used for acute or
chronic bronchitis, whooping cough, and specifically for bronchitis with non-productive cough.(G2, G6, G7, G8, G64)
Figure 1 Selected constituents of white horehound. |
Figure 2 White horehound (Marrubium vulgare). |
361

362 Horehound, White
Figure 3 White horehound – dried drug substance (leaf).
HDosage
Dosages for oral administration (adults) for traditional uses recommended in standard herbal reference texts are given below.
Dried herb 1–2 g as an infusion three times daily.(G6, G7)
Liquid extract 2–4 mL (1 : 1 in 20% alcohol) three times
daily.(G6, G7)
Pharmacological Actions
In vitro and animal studies
Aqueous extracts have been reported to exhibit an antagonistic
effect towards 5-hydroxytryptamine in vivo in mice, and in vitro in guinea-pig ileum and rat uterus tissue.(3) Expectorant and
vasodilative properties have been documented for the volatile oil.(4) However, the main active expectorant principle in white
horehound is reported to be marrubiin, which is stated to stimulate secretions of the bronchial mucosa.(G60) Marrubiin has also been stated to be cardioactive, possessing anti-arrhythmic
properties, although higher doses are reported to cause arrhythmias.(G60) Marrubin acid (obtained from the saponification of
marrubiin) has been documented to stimulate bile secretion in rats, whereas marrubiin was found to be inactive.(5) White horehound is stated to possess bitter properties (BI 65 000
compared to gentian BI 10 000–30 000) with marrubiin as the
main active component.(G62)
Large doses of white horehound are purgative.(G10, G60) The volatile oil has antischistosomal activity.(6)
Clinical studies
There is a lack of clinical research assessing the effects of white horehound and rigorous randomised controlled clinical trials are required.
Side-effects, Toxicity
There is a lack of clinical safety and toxicity data for white horehound and further investigation of these aspects is required.
The plant juice of white horehound is stated to contain an irritant principle, which can cause contact dermatitis.(G51)
No documented toxicity studies were located for the whole plant, although an LD50 (rat, by mouth) value for marrubin acid is reported as 370 mg/kg body weight.(5)
Contra-indications, Warnings
None documented. Cardioactive properties and an antagonism of 5-hydroxytryptamine have been documented in animals. However, the clinical relevance, if any, of these findings is unclear.
Drug interactions None documented. However, the potential for preparations of white horehound to interact with other medicines administered concurrently, particularly those with similar or opposing effects, should be considered.
Pregnancy and lactation White horehound is reputed to be an abortifacient and to affect the menstrual cycle.(G30) Uterine stimulant activity in animals has been documented.(G30) In view of this and the lack of safety data, the use of white horehound during pregnancy and lactation should be avoided.
Preparations
Proprietary multi-ingredient preparations
UK: Rob-Bron Tablets.
References
1Kowalewski Z, Matlawska I. Flavonoid compounds in the herb of
Marrubium vulgare L. Herba Pol 1978; 24: 183–186.
2Henderson MS, McCrindle R. Premarrubiin. A diterpenoid from
Marrubium vulgare L. J Chem Soc 1969; (C): 2014.
3 Cahen R. Pharmacologic spectrum of Marrubium vulgare. C R Soc Biol 1970; 164: 1467–1472.
4Karryev MO et al. Some therapeutic properties and phytochemistry of common horehound. Izv Akad Nauk Turkm SSR Ser Biol Nauk 1976;
3: 86–88.
5Krejcí I, Zadina R. Die Gallentreibende Wirkung von Marrubiin und Marrabinsäure. Planta Med; 1959; 7: 1–7.
6Saleh MM, Glombitza KW. Volatile oil of Marrubium vulgare and its anti-schistosomal activity. Planta Med 1989; 55: 105.
