
Hydrogen compounds
Nitrogen

Ammonia. It is a colourless gas with a suffocating smell. Toxic: irritates the mucous membrane, and|but| a sharp poisoning causes|call| the damage of eyes and lungs.
Structure. According to the hybridization theory the atom of nitrogen in NH3 is in a state of sp3-hybridization.
 The
molecule of NH3
is
a three-cornered pyramid with the atom of nitrogen at the top|
and three atoms of hydrogen at the base. Reason|cause|
of such spatial arrangements|distributing|
of bonds is|appear|
a presence of unshared pair|couple|
of electrons, which|what|
makes a strong repulsive action|act|
on the bonds|truss|:
The
molecule of NH3
is
a three-cornered pyramid with the atom of nitrogen at the top|
and three atoms of hydrogen at the base. Reason|cause|
of such spatial arrangements|distributing|
of bonds is|appear|
a presence of unshared pair|couple|
of electrons, which|what|
makes a strong repulsive action|act|
on the bonds|truss|:
(length of bond – 0.101 nm|, angle - 107о).
Interestingly|curiously|, that stoichiometrically similar |molecule of BF3 is|appear| flat. Boron|it| does not have a lone electronic pair|couple|, which|what| would cause|call| repulsion ||it|on the three two-electronic bonds and break the plane| location|disposition| of BF3.
A remarkable property|virtue| of molecules of NH3 is|appear| their ability|solvency| to|by| the structure inversion, i.e. «turning inside-outside» by passing the atom of nitrogen through a temporary plane formed by atoms of hydrogen located at the base of pyramid.

A potential barrier of this inversion is equal to 25 kJ/mol. Some molecules, which have attained sufficient energy, can realize this process|it| at every instant moment of time. As a result such inversion is related|tied| to the emission of strict certain|definite| frequency (=2.387.1010 s‑1|), based on that phenomena an apparatus was created for a very precise measuring of time. Such «molecular clock» allowed, in particular|including|, to discover that duration of the Earth days annually grows by 0.00043 secs.
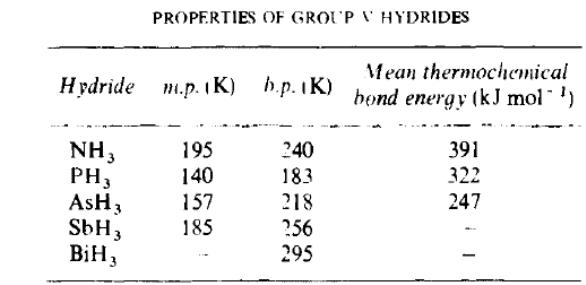
Molecule of NH3 is a strong donor of electronic pair|couple| and is characterized by|described| high polarity ( = 1.46 D), as electrons of bonds of N—H are strongly displaced from| H to|by| N. Polarity of the bond N—H explains |condition,cause| hydrogen bond formation between the molecules of NH3. The presence of hydrogen bonds in liquid|rare,thin| and solid ammonia explains many of its|its| extraordinary properties|virtue| in compared to other hydrogen compounds|halving,compound,junction,joint,coupling| of V group elements. For this reason m.p. (-77.75 0С) and b.p. (-33.420) of NH3 are comparatively high, it is characterized|described| by considerable enthalpy of evaporation and ammonia is liquefied| easily. That property of ammonia is thereon us|utillizing|ed in the machines|vehicle| of refrigerations.

Preparation.
In industry (synthesis process by Fritz Haber). The process involves direct combination of N2 and H2 under special conditions:
N2
+
3H2
![]() 2NH3
= -92.4
kJ/mol
2NH3
= -92.4
kJ/mol
(catalyst - Fe, 400-5000, pressure 1000 atm).

In a laboratory:
NH4Cl + Ca(OH)2 CaCl2 + NH3 + H2O
Chemical properties|virtue|. By solubility in water NH3 exceeds any|some| other gas: at 0оС one 1 volume of water absorbs 1200 volumes of gaseous NH3 and|but| 700 more volumes at 200С. Wonderful solubility is based on the formation of intermolecular hydrogen bonds. Two mechanisms of the formation of hydrogen bonds are thus possible between the molecules of NH3 and water. Since donor| property|solvency| of a molecule of NH3 is expressed stronger than in water, and|but| the bond О—Н| is more polar in compared with the polarity of bond of N—H in NH3, and the intermolecular| hydrogen bond forms according thethe first mechanism.
Thus|on this grow|, physical and chemical processes in water|aquatic| solution of NH3 can be presented in such form|appearance|:

The formation of ОН-| ions creates an alkaline reaction in the solution of NH3 in water. The constant of ionization is low (К = 2.10-5), preving that the equilibrium of this reaction is strongly displaced to the left (in 1М solution ammonia hydroxide is ionized only 0.4% NH3 and Н2О). The commercial|corrupt| concentrated solution of NH3 usually has density|closeness,thickness| of 0.91 g/cm3 and contains|maintain| 25 mass| % NH3 (that approached the composition of NH3.3Н2О). At cooling of solutions of NH3 can be isolated crystalhydrates| of NH3.H2O, m.p. = -77оС), 2NH3.H2O (m.p. = -78оС), NH3.2H2O (m.p. = -97оС), which|what| consist of chains of molecules of NH3 and H2O, bound together by hydrogen bonds|truss| in which molecules of NH4OН are absent|what|. That is why|that is why| although solution of NH3 in water is named|called| “ammonium hydroxide” this name is conditional, and a molecule of NH4OH as an individual chemical does not exist. Thus|on this grow|, aqueous solutions of NH3 have basic|main| characteristics not due to the formation of mythical compound|halving,compound,junction,joint,coupling| of NH4OH, but because of|because of,owing to| the extraordinarily expressed donor| activity of atom of nitrogen in NH3.
For NH3 are characteristic|character,typical| reactions of three types|appearance|:
1. reactions of addition. An interaction of NH3 with acids gives salts of ammonium:
NH3 + H+ = NH4+
NH3 + HCl = NH4Cl
(interestingly|curiously|, that reaction does not proceed|leaves| at complete absence of water!!!)

The ion of NH4+ is and| most of its|its| salts are colourless. In the solid state|figure,camp,mill| the salts of ammonium form structures, characteristic|character,typical| of compounds with the considerable part|stake,share| of ionic bond. That is why|that is why| they are well dissolved in water and dissociate| almost completely. The structure of NH4+ is tetrahedral|, in which|what| the peaks| of tetrahedron are occupied by the atoms of hydrogen, and|but| an atom of nitrogen is in the center.
A positive|staid| charge is evenly distributed between all atoms of hydrogen.
Ammonium salts at hydrolysis give acidic medium|Wednesday|
NH4+ + H2O = NH4OH + H+
as NH4OH is a weak base|foundation|.
Salts of ammonium are stable|firm| under ordinary|usual| conditions, but at heating are decomposed. Nature of the final|end| products product of thermal decomposition of ammonium salts is mainly determined by the properties|virtue| of anion|.

If an acid residue is a strong oxidant |remainder|, ammonium nitrogen is oxidized: in such reaction ammonium nitrogen gives|return| 4 electrons to nitrate anion nitrogen which|what| is|appear| an oxidant. On the other hand|on other hand|, this reaction is an example of intramolecular disproportionation|.
Other examples are:
NH4NO2 = N2 + 2H2O
(NH4)2Cr2O7 = Cr2O3 + N2 + 4H2O
3(NH4)2SO4 = N2 + 3SO2 + 6H2O + 4NH3
If an acid is|appear| not an oxidant, the type|nature| of decomposition depends on its volatility at temperature of decomposition:
1. From the salts of non-volatile acids is eliminated only NH3:
(NH4)3РO4 = 3NH3 + H3РO4
2. If an acid is volatile|flying| (for example HCl)
NH4Cl = NH3 + HCl
at cooling it again forms an initial|output| salt and process of decomposition looks like sublimation of salt.
In liquid|rare,thin| ammonia exist dimers (NH3)2 which|what| are able to|by| selfdissociate. However, this process is |it|extraordinarily moved to the left (at -50оС):
(NH3)2 = NH4+ + NH2- К = 2.10-33
2. reactions of substitution. The reactions of hydrogen substitution of ammonia are less characteristic|character,typical|, than the reactions of addition. They proceed at high temperatures. At substitution of one hydrogen in NH3 it forms amides of metals MeNH2, at substituting of two atoms of hydrogen –imides of | metals Me2NH, at substituting of three atoms of hydrogen – nitrides of| metals.
Amides. Dry ammonia interacts with metals (in fusions):
2![]() +
2Na = 2NaNH2
+
+
2Na = 2NaNH2
+
![]()
= -145 kJmol NaNH2-
amide|
= -145 kJmol NaNH2-
amide|
Thus, here hydrogen of NH3 lowers its oxidation state while NH3 acts as an oxidant. On the other hand|on other hand|, this reveals| the acidic nature of NH3|virtue|. Amides of metals are|appear| typical|model| salts of NH3, which|what| correspond to its|its| acidic function. Acid nature of NH3 is expressed very poorly, the constant of acid ionization extraordinary low (рКа 35), that is why|that is why| salts of NH3 as acids in water hydrolyse| completely:
NaNH2 + H2O = NaOH + NH3
Imides. Dry ammonia interacts with metals (at fusion):
NH3 + 2Li = Li2NH + H2; Li2NH- lithium imide
Imides also can be prepared|received| by careful heating of amides|:
2LiNH2 = LiNH + NH3
Imides also correspond to the acidic function of ammonia.
Nitrides. At annealing| of solid Al in the atmosphere of NH3 occurs a reaction:
2Al + 2NH3 = 2AlN + 3H2
this process of preparation of nitride| coating films is called nitridation|.
Nitrides of metals, unlike halides or| sulfides, are|appear| not salts, as they do not correspond to any|some,any| acids. They are formed at high temperatures:
3Mg + N2 = Mg3N2
2B + N2 = 2BN
Properties|virtue| of nitrides| more or less appropriately change according periods and groups of the periodic system. For example, in small periods there is observed a transition from| basic|main| nitrides |to|by| acidic ones:
Na3N Mg3N2 AlN Si3N4 P3N5 S4N4 Cl3N
basic|main| amphoteric| acidic
Nitrides are classified so: ionic (Li3N), covalent| (NH3) and “inclusion”|inclusion| (Fe4N, Fe3N). Nitrides of| s-elements have mostly ionic type of bonds|truss|, oxidation state of nitrogen there is -3. These nitrides| are crystalline compounds. They are chemically active, easily react with water:
Mg3N2 + 6H2O = 3Mg(OH)2 + 2NH3
Nitrides BN, AlN, Si3N4 are polymeric compounds with high temperatures of melting (2000-3000оС), they are dielectrics or semiconductors.
Nitrides of d-metals (Fe4N, Fe3N, Ni3N) are compounds|halving,compound,junction,joint,coupling| with metallic bond, they have metallic (electronic) conductivity. They are products of squeezing of atoms of nitrogen into the holes of crystalline grate of metals. Filling of these empty space account for material strengthening, those nitrides are remarkably hard |, refractory|that is why|, chemically inactive, not reacting with water, only slowly|sluggishly| reacting with acids. The oxidation state of elements in them is accepted as equal to|equal| zero.
It is obvious, that from the covalent| nitrides| a greatest practical value|importance,meaning| has nitride| of hydrogen of NH3- ammonia. Next to derivatives of metals are known the products of substituting of hydrogens of ammonia on a halogen, for example chlorous nitrogen of NСl3, which|what| forms as yellow oily drops obtained at|receive|action act|act| action of Cl2 on the concentrated solution of NH4Cl:
NH4Cl + 3Cl2 = NCl3 + 4HCl (1HCl + 3HCl)
NСl3 is very unstable, at heating to|by| 90оС or at slight blow|kick| it decomposes with an explosion on elements. In water NСl3 is not dissolved, but slowly|sluggishly| hydrolysed|it|:
NCl3 + 3H2O = NH3 + 3HOCl
3. Reactions of oxidation. The oxidation state of nitrogen (-3) in NH3 is|appear| lowest, however |virtue| its reduction |its| activity is expressed very poorly: NH3 does not burn|burning| in air, it does not react in solutions with most oxidants, for example with compounds|halving,compound,junction,joint,coupling| of Cr(VI). It is explained with the fact that NH3 and ion of NH4+ are comparatively stable molecules|firm|.
However NH3 normally burns|burning| in the atmosphere of oxygen:
4NH3 + 3O2 = 2N2 + 6H2O
and|but| in the presence of catalysts in the air:
4NH3 + 5O2 = 4NO + 6H2O (basic|main| process of HNO3production)
Cl2 and Br2 oxidize ammonia vigorously:
2NH3 + 3Cl2 = N2 + 6HCl
(this reaction proceeds also in solutions).
Ammonia interestingly|curiously| reacts with J2.Thus forms extraordinarily unstable compound NI3, which|what| |appear|, when it is dried up, violently explodes from| a least touch («chemical inviolability»).
NH3 can reduce oxides|oxide|:
2NH3 + 3CuO = 3Cu + N2 + 3H2O
On the whole|all in all| the properties|virtue| of NH3 can be presented in such a chart:

Hydrazine, N2H4. It is a colourless toxic liquid which|what| easily evaporates (tboiling = 113,50С), it has high dielectric permeability ( = 52 at 25оС), it is polar (=1.85 D).
The structure|building| of N2H4 is similar|like| to|by| H2O2 (the so-called|so called| goch-configuration|shape|):

Trans- goch-
More symmetric trans-form|shape| does not answer reality, as N2H4 has high dipole moment (1.85D), greater than in water, which|what| can not be demonstrated|shown,turned,displayed| at the symmetric trans form|shape|.
Preparation. In laboratory:
2NH3 + NaClO = N2H4 + NaCl + H2O
Chemical properties|virtue|. Chemical properties|virtue| of N2H4 look a great deal similar to those of ammonia. In its|its| aqueous|aquatic,water| solutions also exist hydrogen bonds|truss|. Basic|main| properties|virtue| of N2H4 are related|tied| to the mobility of electronic pair|couple| on the atom of nitrogen. Being a donor of two electronic pair|couple|, in an acidic medium|Wednesday| hydrazine adds protons:
NH2NH2 + H+ = NH3+NH2 К1 = 8.5.10-7
NH3+NH2 + H+ = NH3+NH3+ К2 = 8.5.10-15
As a result salts of |hydrazine are known as follows |that is why|:
N2H4 + HCl = [N2H5]Cl = N2H4.HСl
N2H4 + 2HCl = [N2H6]2+Cl2
Cation [N2H6]2+ is stable|firm| only at large|great,big| excess|overabundance| of strong acid, in other case it is|its| completely hydrolysed:
NH3NH32+ + H2O NH3NH2+ + H3O+
The atom of nitrogen in N2H4 is characterized|described| by Red-Ox duality:
demonstrates |virtue| reductive properties;
N2H4 + O2 = N2 + 2H2O
3N2H4 = N2 + 4NH3
N2H4 + 2I2 = N2 + 4HI
N2H4 + 2Cl2 = N2 + 4HCl
5N2H4 + 4KMnO4 + 6H2SO4 = 5N2 + 4MnSO4 + 2K2SO4 + 16H2O
much weaker are expressed its oxidizing properties|virtue|
N2H4 + 2H+ = 2NH3
(Zn+HCl)
Hydroxylamine, NH2OH. It is a solid white compound, m.p. = 33 oС. Thermally unstable; at higher than 100оС it explodes. Its|its| aqueous solutions are more stable|firm|.
Preparation. Electrolysis on cathode at reduction of HNO3 solution by atomic hydrogen gives NH2OH
HNO3 + 6H+ + 6е = NH2OH + 2H2O

An atom of nitrogen in NH2OH is a donor of electronic pair|couple|, that is why|that is why| NH2OH forms a drogen bond. Like NH3 and N2H4 in water of NH2OH is a weak base|foundation|. In the series NH3 (К = 10-5) N2H4 (К = 10-6) NH2OH (К = 10-8) basic|main| properties|virtue| diminish.
With acids NH2OH forms salts according to reaction of addition type:
NH2OH + HCl = [NH3OH]Cl
Chemical properties|virtue| are similar to the properties|virtue| of NH3 and N2H4.
NH2OH is easily decomposed as a result of reaction of disproportioning|:
3NH2OH = NH3 + N2 + 3H2O
The atom of nitrogen in NH2OH has an oxidation state (-1), that is why|that is why| it is simultaneously a reductant and oxidant.
Hydroxylamine demonstrates reductive properties|virtue|:
NH2OH + K2Cr2O7 + 4H2SO3 = 3N2 + Cr2(SO4)3 + K2SO4 + 13H2O
2NH2OH + I2 + 2KOH = N2 + 2KI + 4H2O
4NH2OH + O2 = 2N2 + 6H2O
As well as oxidizing properties|virtue|:
2NH2OH + 4FeSO4 + 3H2SO4 = 2Fe2(SO4)3 + (NH4)2SO4 + 13H2O
NH2OH + 3HI = I2 + NH4I + H2O
Hydrogen azide, HN3. It is a colourless volatile|flying| liquid, tboiling = 370С, with a strong smell.
The least negative oxidation state of nitrogen (-1/3) is realised in HN3 among the other hydrogen compounds|halving,compound,junction,joint,coupling| of nitrogen. This unusual oxidation state is determined by structural unequivalence of atoms of nitrogen. From a position of the Valence bond method | this unequivalence can be given by a chart:

The major point |head,leading| in this chart is delocalisation of | - bonds along the line which|what| connect the atoms of nitrogen. A correctness of the scheme is|proved,argued| confirmed by the value of distances between the atoms of nitrogen 1—2 and 2—3, which|what| are|appear| intermediate between the lengths of bonds -N=N- and NN. Atoms of nitrogen in the molecule of HN3 are in the state|figure,camp,mill| of sp-hybridization, that is why|that is why| the ion of N3- has a linear structure.
Preparation. Water|aquatic| solution of HN3 has name| hydroazoic| acid. It is prepared according to the reaction: N2H4 + HNO2 = HN3 + 2H2O
Chemical properties|virtue|. It is weak acid (~ CH3COOH)
HN3 = H+ + N-3 К = 2.10-5
At heating of vapor|couple| of HN3 higher to 300оС it|it| is decomposed with an explosion|decomposed|:
2HN3 = H2 + 3N2
In the waterless state|figure,camp,mill| HN3 explodes even at shaking of a vessel, in the diluted state|figure,camp,mill| it is stable, as the reaction of its decomposition
HN3 + H2O = N2 + NH2OH (disproportioning|)
proceeds|leaves| extraordinarily slow|sluggishly|.
Reductive properties of HN3 are not known|character,typical|, however there is a single reaction:
HN3 + HNO2 = N2 + N2O + H2O
Oxidizing properties|virtue| of HN3are characteristic|character,typical| (strong oxidant!!):
HN3 + 3HCl = Cl2 + N2 + NH4Cl
(N.N2)
The mixture of HN3 with concentrated HСl dissolves|opens| Au and Pt, that similar aquafortis. According the oxidizing reactions HN3 is similar to those of HNO3 as a result of|because of,owing to| presence of four-covalent| nitrogen in their molecules:
Cu + 3HN3 = Cu(N3)2 + NH3 + N2
Salts of HN3 have name|called| azides|. An initial|output| product for the preparation of azides| is|appear| NaN3, which|what| can be prepared|received|:
NaNH2 + NO2 = NaN3 + H2O
Azide| of lead Pb(N3)2 is used in detonators.
Phosphorus
Phosphorus compounds with H. Phosphorus practically does not react with H2. The process:
2P + 3H2 2PH3 Но = -12.6 kJ / mol
p roceeds
very slowly. At 350 oC
and 200 atm PH3
yield
is close to 2% and the equilibrium under these conditions is
established only in 6 days. Therefore, hydrogen
compounds of phosphorus are usually obtained indirectly.
roceeds
very slowly. At 350 oC
and 200 atm PH3
yield
is close to 2% and the equilibrium under these conditions is
established only in 6 days. Therefore, hydrogen
compounds of phosphorus are usually obtained indirectly.
There are a few hydrogen compounds of P. The most studied is phosphine PH3, and the so-called “liquid” P2H4 and “solid” P4H2 hydrogen phosphides.
Phosphine. This is a gas with a characteristic unpleasant smell. Pure phosphine is odourless, but technical grade phosphine has a highly unpleasant smell like garlic or rotten fish, due to the presence of substituted phosphine and diphosphine, P2H4. It is a very strong poison, which is a major obstacle to its practical application. Poisoning PH3 damages nervous system. MAC PH3 in air is 0.00001 mg / l.
Preparation. The most frequently used is the heating white P with concentrated aqueous solution of alkali:
Р4 + 3NaОН + 3Н2О = РН3 + 3NаН2РО2
