
2 Nomenclature of Hydrocarbons
Nomenclature: naming of compounds
There are three tips of nomenclature: trivial, rational and IUPAC (systematic)
Trivial nomenclature uses parent names, that were formed historically.
e.g.
acetic acid, tartaric acid, wood alcohol.

Accoding to the rational system of nomenclature, organic compounds are the derivatives of the simplest member of homologous series hydrocarbons: alkanes – methane, alkenes – ethane (ethylene), alkynes – ethyne (acetylene)

Rational system of nomenclature is used not often (for naming of compounds with simple structure).
IUPAC system of nomenclature has the largest use
The formal system of nomenclature is proposed by the International Union of Pure and Applied Chemistry (IUPAC)
Fundamental principle of IUPAC system of nomenclature: Each different compound should have a different name.
Straight-Chain Acyclic Hydrocarbons
1. The naming of organic compounds is based on the parent hydrocarbon they derived from. The number of carbon atoms in hydrocarbons is represented by stem names.
Number of carbon atoms |
Stem name |
|
Number of carbon atoms |
Stem name |
1 2 3 4 5 |
Meth- Eth- Prop- But- Pent- |
|
6 7 8 9 10 |
Hex- Hept- Oct- Non- Dec- |
2. (a) The suffix ‘ane’ is used for saturated hydrocarbons.
e.g. CH3 − CH3 ethane CH3CH2 − CH3 propane
(b) The suffix ‘ene’ is used for unsaturated hydrocarbons with a double bond.
e.g. CH2 = CH2 ethene CH3CH = CH2 propene
(c) The suffix ‘yne’ is used for unsaturated hydrocarbons with a triple bond.
e.g. CH º CH ethyne CH3CH º CH propyne
3. The lowest possible number is assigned to the carbon atoms of the multiple bond.
The number is written before the suffix to indicate the position of the carbon atom of the multiple bond
e.g. CH3CH2CH = CH2 but-1-ene (not but-3-ene)
CH3C º CCH2CH3 pent-2-yne (not pent-3-yne)
4. If a compound contains more than one double or triple bond, the prefixes like ‘di-’, ‘tri-’, are used to indicate its number of occurrence. An ‘a’ is added to the corresponding stem name.
e.g. CH3CH2CH = CHCH = CH2 hexa-1,3-diene
5. If a double or triple bond is not named in the ending of a name, the suffix ‘-en-’ and ‘-yn-’ are used respectively in the name.
e.g. CH2 = CHOH ethenol
6. The geometric isomers of an alkene are specified by the word of ‘cis-’ or ‘trans-’ before their names. e.g.


Branched-Chain Acyclic Hydrocarbons
1. Select and name the parent hydrocarbon (a) For saturated hydrocarbons,
Parent chain: longest possible straight chain branched chain: all remaining e.g,
![]()

(b) For unsaturated hydrocarbons,
Parent chain: longest possible straight chain which contains the maximum number of multiple bonds
(c) The parent hydrocarbon is named as described in the naming of straight-chain acyclic hydrocarbons
Name the branched chains
(a) The number of carbon atoms in a branched chain is indicated by the stem names
(b) The suffix ‘-yl’ is added to the corresponding stem names for branched chains containing only single bonds.
e.g. – CH3 Methyl
– CH2CH3 Ethyl – CH2CH2CH3 Propyl
 Isopropyl
or sec-Propyl
(secondary)
Isopropyl
or sec-Propyl
(secondary)

Butyl
sec-Butyl (for secondary carbon, 20)
Isobutyl
tert-Butyl (for tertiary carbon , 30)
3. Numbering
carbon atoms in the parent hydrocarbon
(a) For
saturated hydrocarbons, the parent hydrocarbon is numbered beginning
with the end of the chain nearer the branched chain. e.g.

(b) For unsaturated hydrocarbons, the parent hydrocarbon is numbered so as to include both carbon atoms of the double or triple bond. The numbering begins with the end of the chain nearer the double or triple bond.
e.g.


4. Use the numbers to designate the position of the branched chain.
The parent name is placed last, and the branched chain, preceded by the number designating its position on the parent chain, is placed first. e.g.




5. When two or more branched chains are present, a number corresponding to its position on the parent hydrocarbon is given to each branched chain so as to give the lowest possible numbers to the branched chains. The branched chains are listed alphabetically (i.e. ethyl before methyl). Multiplying prefixes such as ‘di’ and ‘tri’ are ignored when deciding the alphabetical order. e.g.

6. When two branched chains are present on the same carbon atom, use that number twice. e.g.
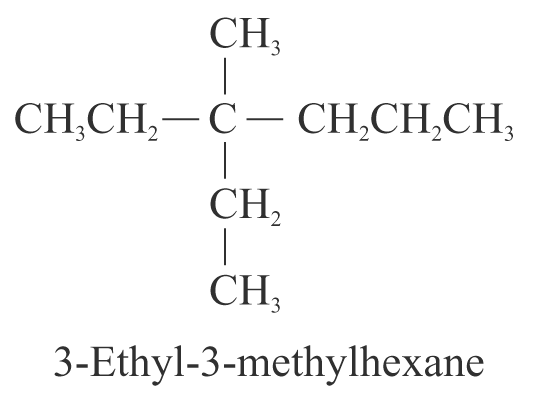
7. When two or more branched chains are identical, indicate this by the use of the prefixes ‘di-’, ‘tri-’, ‘tetra-’, and so on. Commas are used to separate numbers from each other. e.g.



Cyclic Hydrocarbons
Simple cyclic hydrocarbons are named by adding prefix ‘cyclo-’ to the names of their corresponding acyclic counterparts. e.g.




Aromatic Hydrocarbons
1. Some aromatic hydrocarbons possess specific names.



2. In substituted benzenes, the benzene ring is numbered so as to give the lowest possible numbers to the substituents. When more than two substituents are present and the substituents are different, they are listed in alphabetical order. e.g.


