
Lecture 5
Aliphatic compounds (2)
Alkenes (continued)
Alkynes
Dienes
Alkenes (continued)
Addition of Bromine
Alkenes react rapidly with Br2 in 1,1,1-trichloroethane at room temperature and in the absence of light
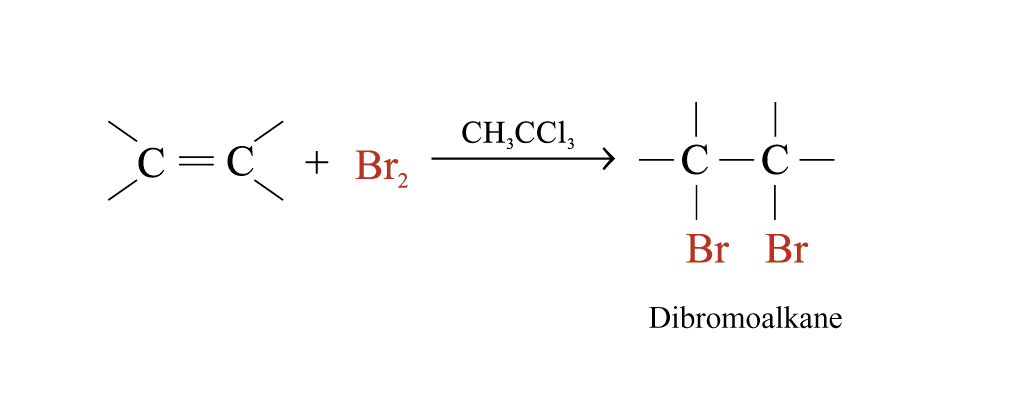
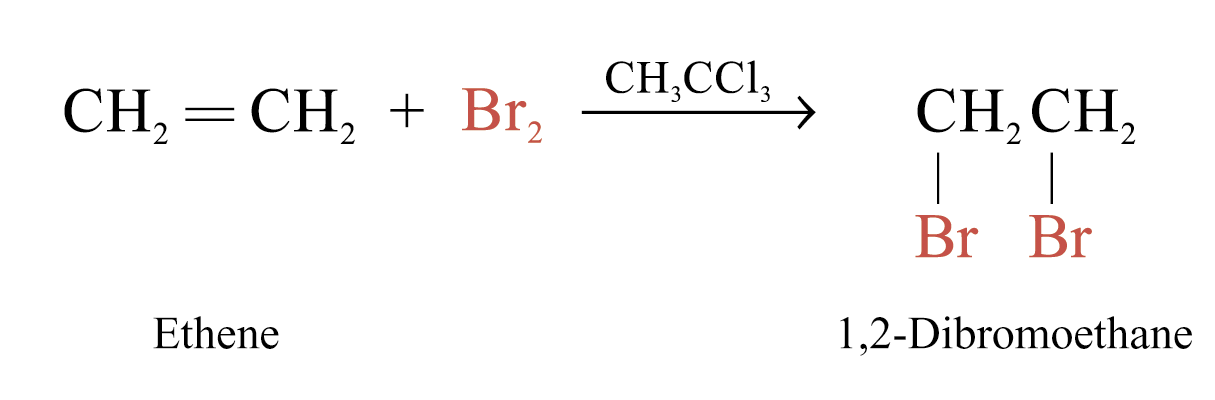
The behaviour of alkenes towards Br2 in CH3CCl3 is a useful test for the presence of carbon-carbon multiple bonds
Addition of Bromine Water
In an aqueous solution of Br2, the following equilibrium exists
Br2 + H2O → HBr + HOBr Bromic(I) acid
The bromine atom bears a partial positive charge while the oxygen atom bears a partial negative charge ∵ oxygen is more electronegative than bromine
![]()
When bromic(I) acid reacts with alkenes, bromohydrin is formed
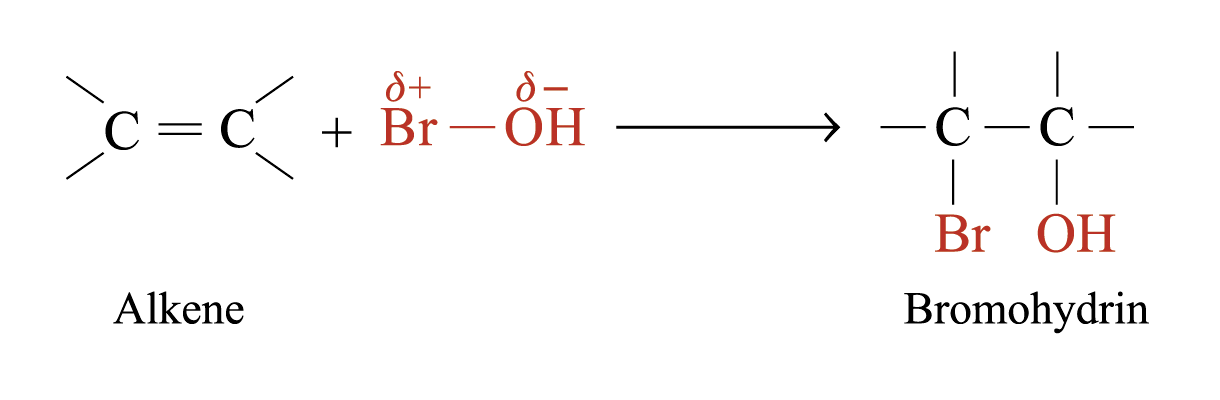
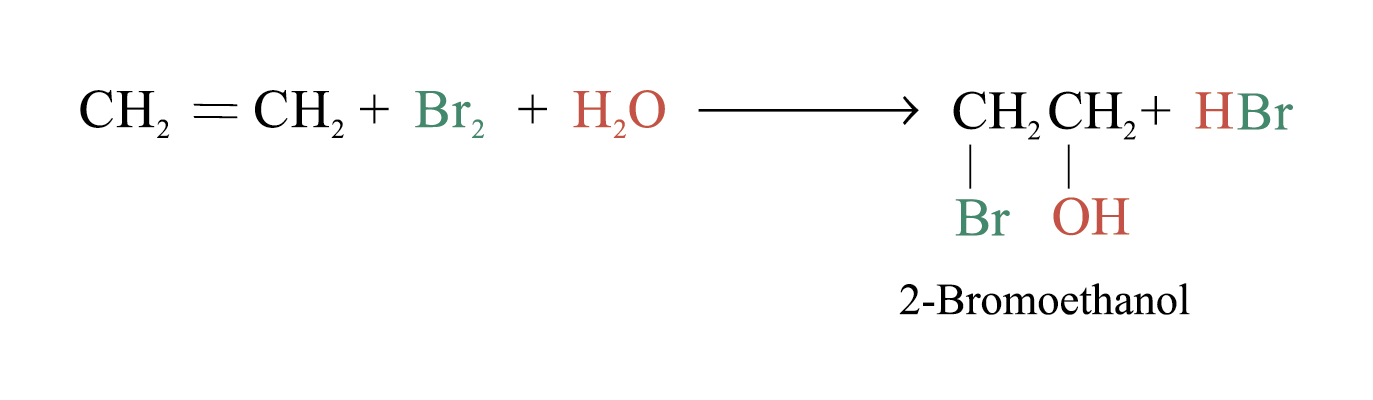
Addition of Sulphuric(VI) Acid
Alkenes react with cold and concentrated H2SO4 to form alkyl hydrogensulphates
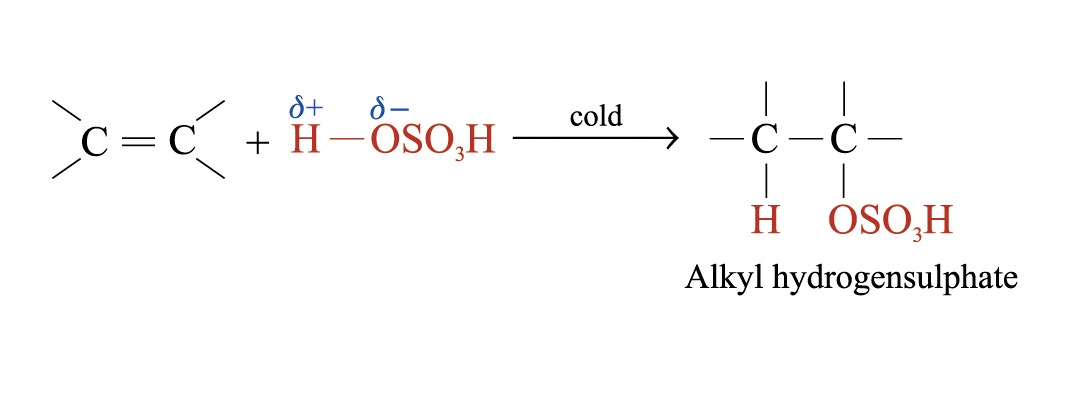
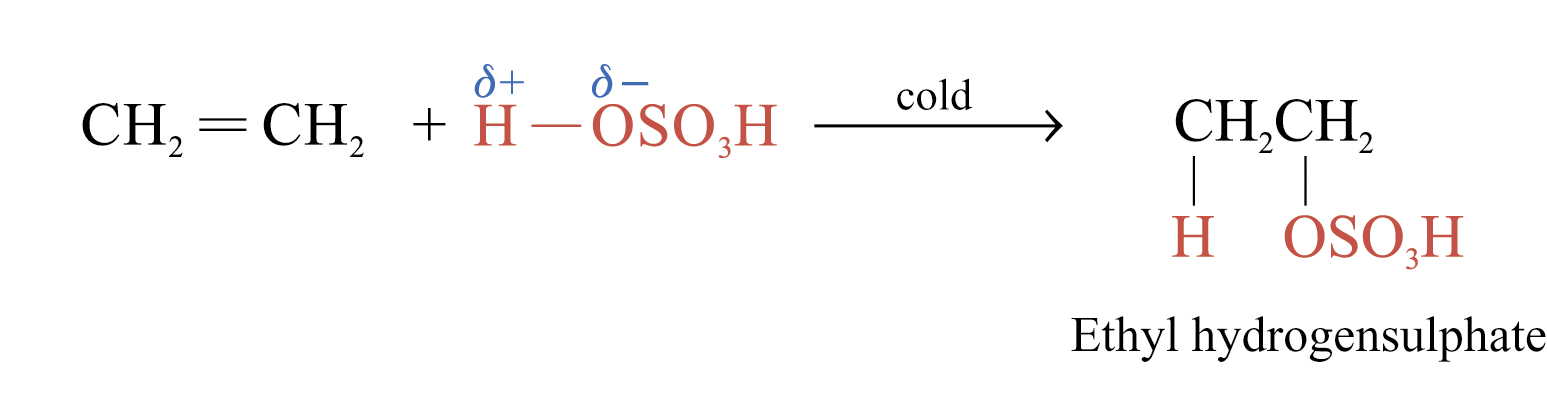
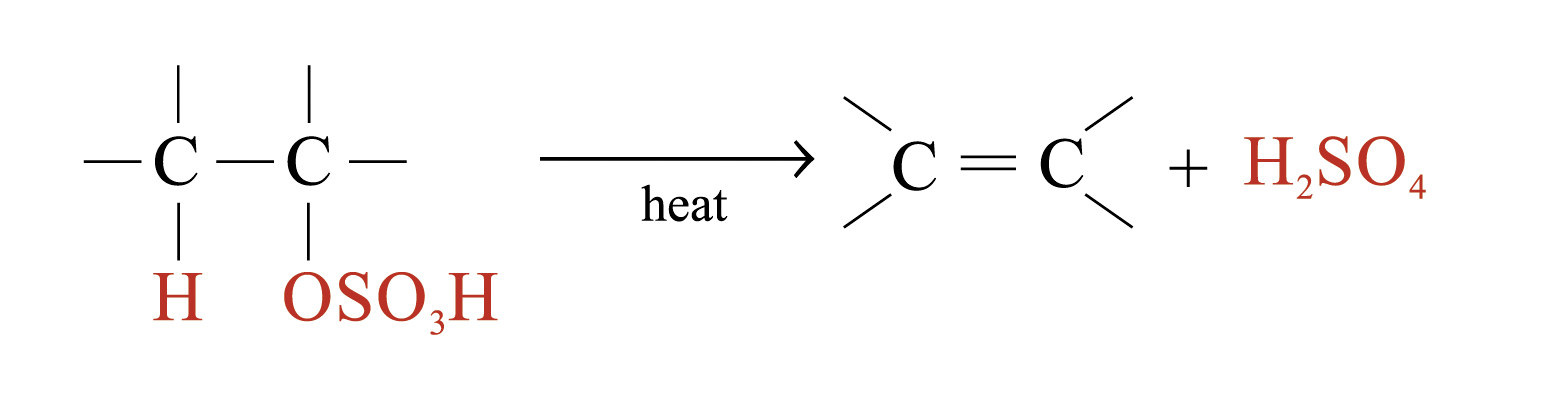
The large bulky –OSO3H group makes the alkyl hydrogensulphate very unstable. Two possible further reactions take place:
1. Regeneration of alkenes
2. Production of alcohols
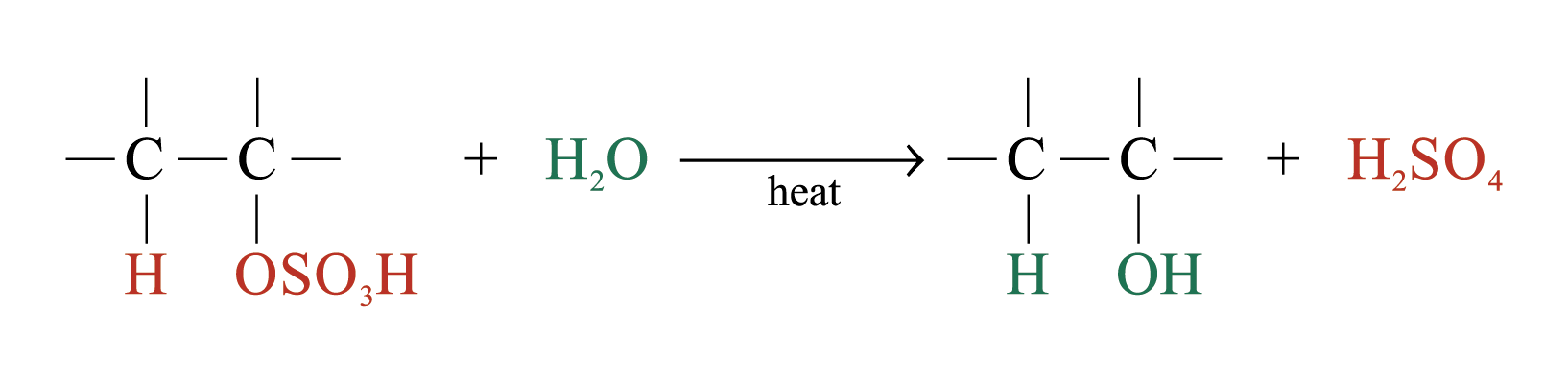
Catalytic Hydrogenation
In the presence of metal catalysts (e.g. Pt, Pd or Ni), H2 is added to each atom of C = C double bond to form an alkane
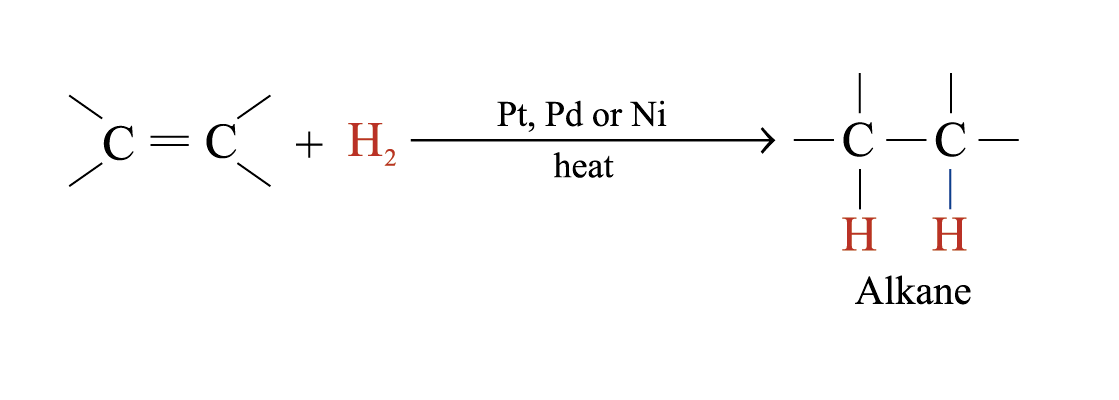
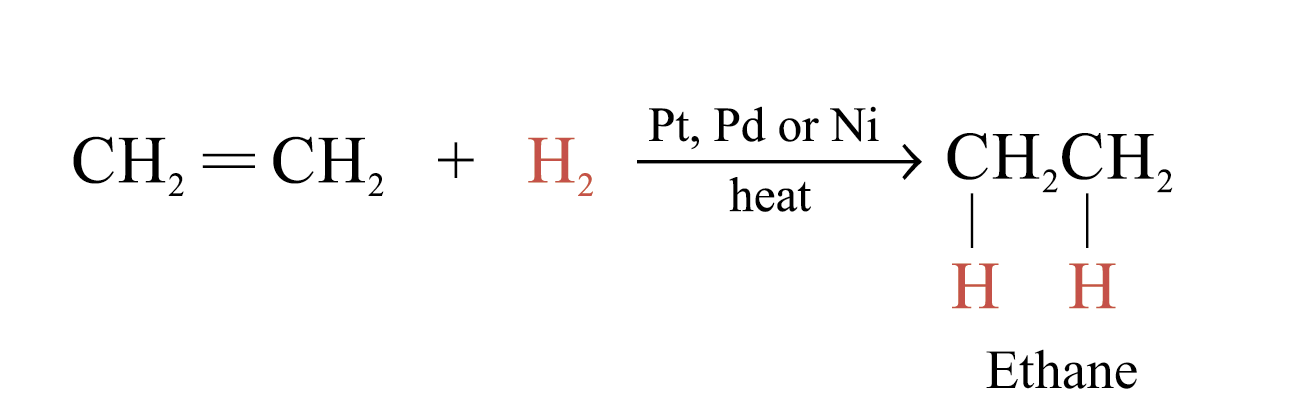
Hydrogenation is useful in analyzing unsaturated hydrocarbons
The number of double or triple bonds present in the unsaturated hydrocarbon molecule can be deduced by the number of moles of hydrogen reacted
Catalytic hydrogenation is used to convert liquid vegetable oil to semi-solid fats in making margarine and solid cooking fats
Oxidation
As a result of reaction of alkenes and diluted KMnO4 (Vagner reaction) diols are formed (decolorization of diluted KMnO4 is the qualitive reaction of multiple bond)
![]()
When alkenes react with powerful oxidizing agents such as ozone or KMnO4, under the high temperature, double bond is broken and aldehydes, ketones, and carboxylic acids are formed.
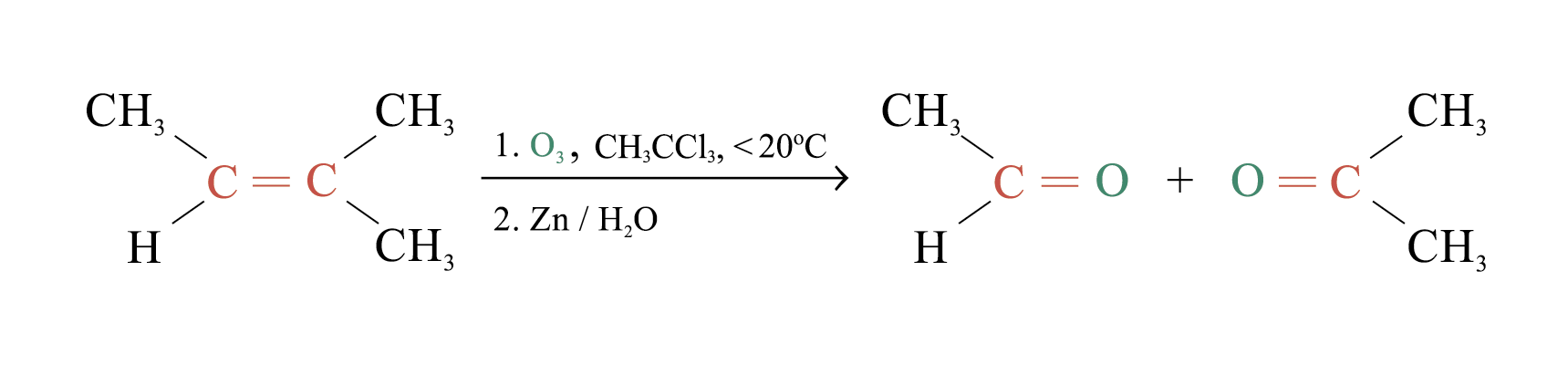
Ozonolysis
Ozonolysis is a widely used method for locating the double bond of an alkene
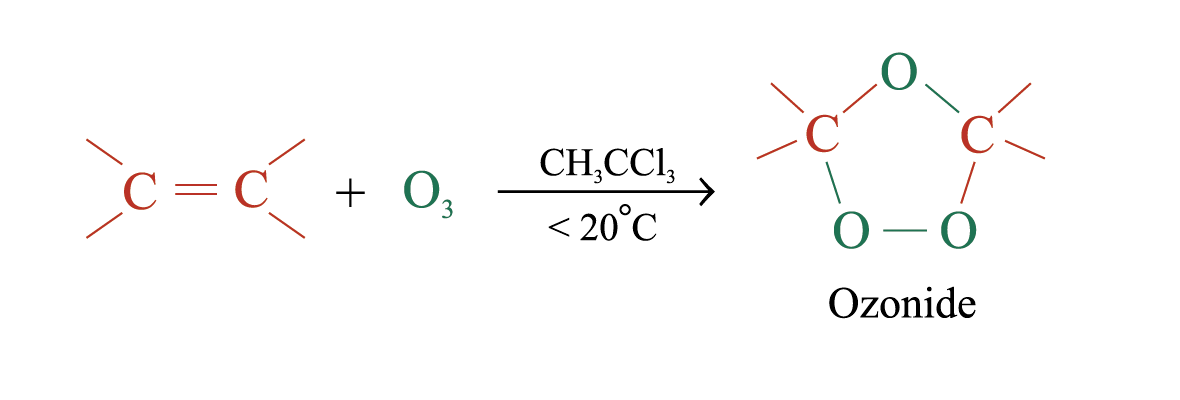
(unstable)
The unstable ozonide is reduced directly by treatment with Zn and H2O
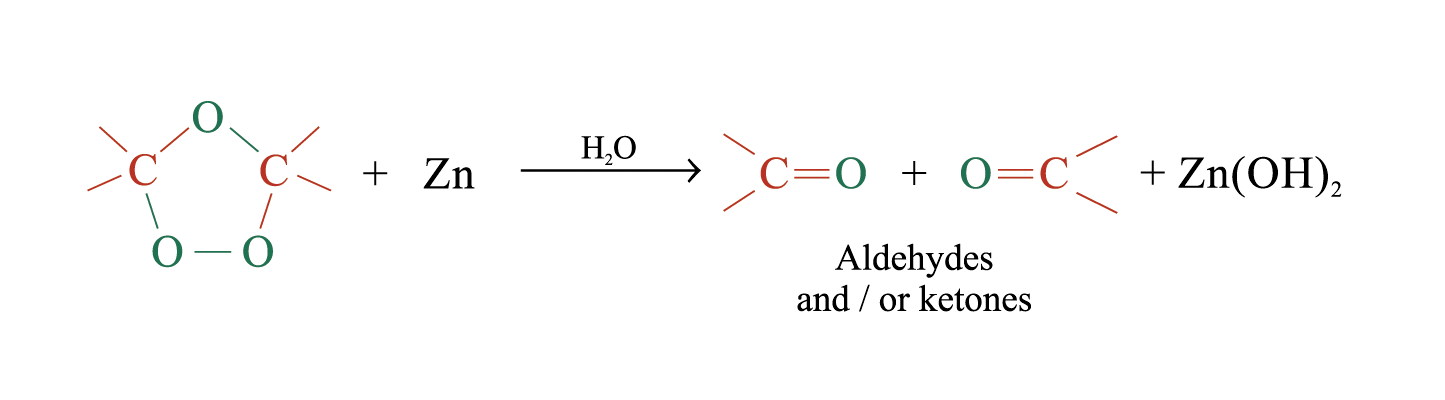
Overall process of ozonolysis:
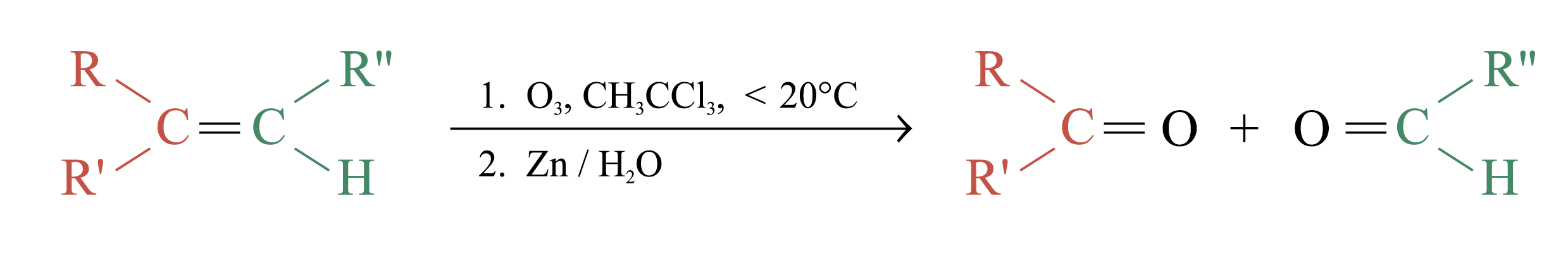
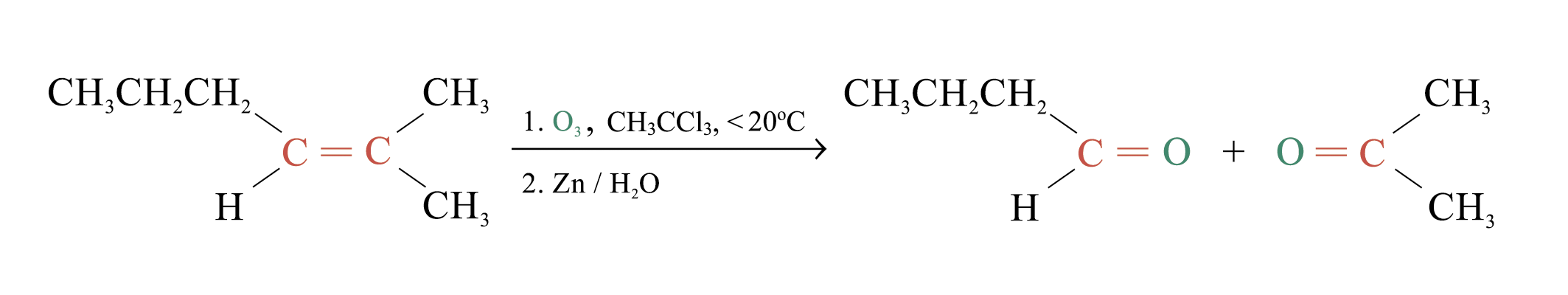
Polymerization
Polymers: Compounds that consist of very large molecules made up of many repeating units
Monomer: Each repeating unit
Polymerization: The reaction by which monomers are joined together
Addition polymerization: alkene monomers are joined together without the elimination of small molecules
Addition polymer: The polymer produced by addition polymerization
Poly(ethene)
Monomer: ethene
Depending on the conditions, two kinds of poly(ethene) are formed
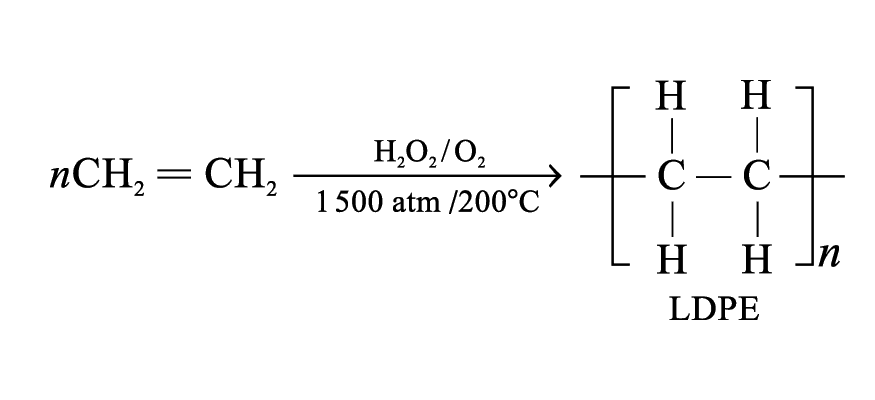

Low density poly(ethene) (LDPE):
Molecular mass: 50 000 to 3 000 000
Light, flexible and low melting temperature
Uses: make soft items like wash bottles, plastic bags and food wraps
High density poly(ethene) (HDPE):
Molecular mass: up to 3 000 000
Tougher and higher melting temperature
Uses: make more rigid items like milk bottles and water buckets
Poly(propene)
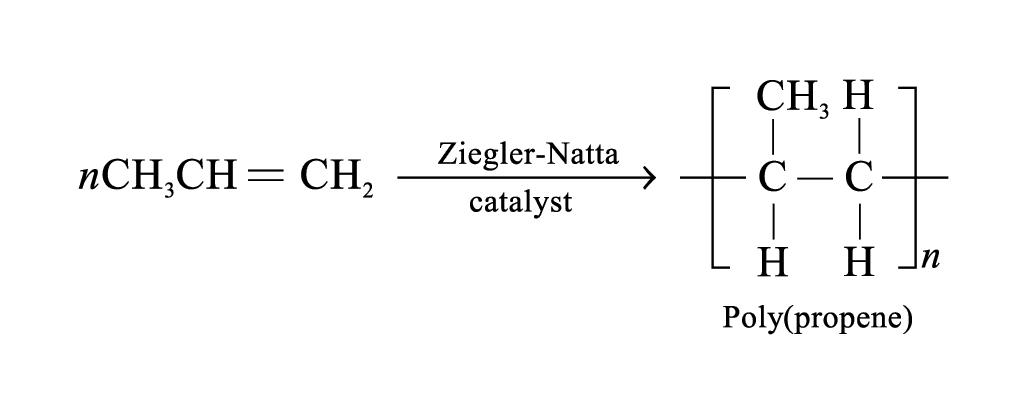
Properties: more rigid than HDPE, high mechanical strength, strong resistance to abrasion
Uses: make moulded furniture; make crates, kitchenware, food containers; make ropes and hard-wearing carpets
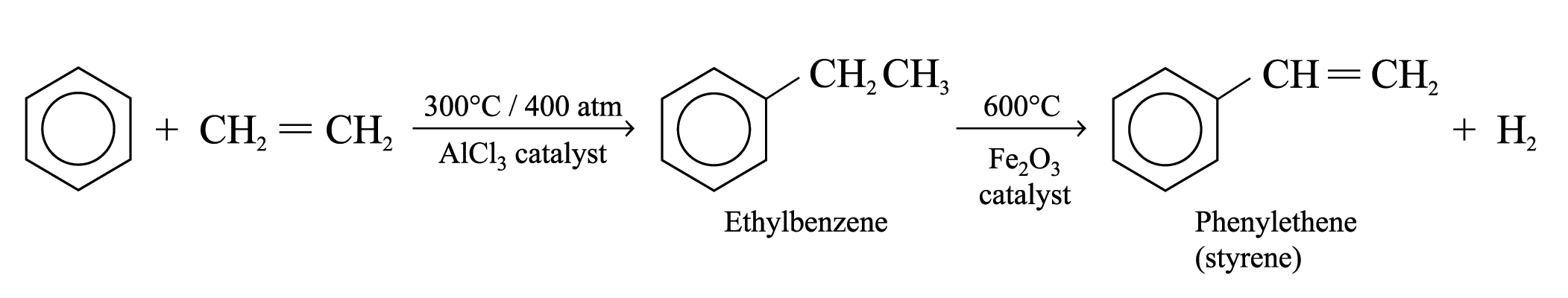
Poly(phenylethene) (or Polystyrene)
Preparation of monomer (phenylethene):
Formation of poly(phenylethene):
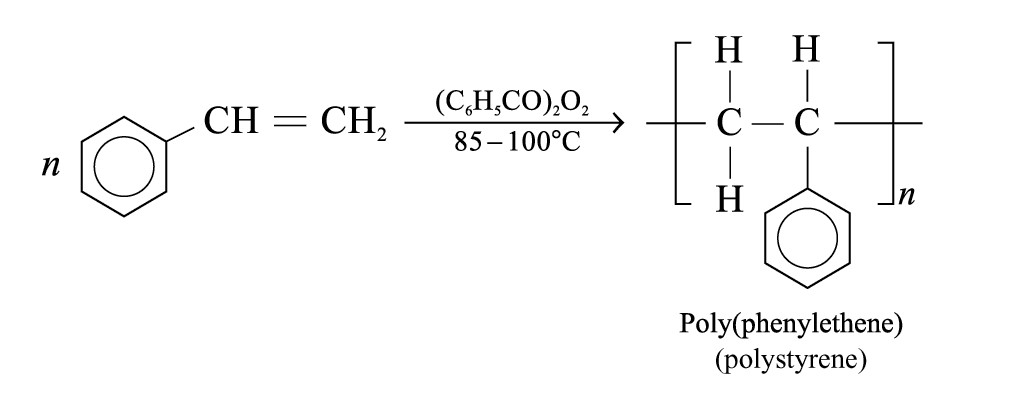
Poly(phenylethene):
Properties: Transparent, brittle and chemically inert
Uses: Make toys, specimen containers and cassette cases
