
- •Types of Organic Reactions
- •Isomerism
- •Types of Organic Reactions
- •1.1 Classification of organic reactions by type of chemical bonds braking
- •Classification of organic reactions by nature of reagent.
- •Classification by the type of organic reactions
- •Classification by number of molecules, which take part in the slowest step of organic reaction (by order reaction)
- •Isomerism
- •2.1. Structural Isomerism
- •Stereoisomerism
Lecture 2
General Organic Chemistry (2)
Types of Organic Reactions
Isomerism
Structural Isomerism
Stereoisomerism
Types of Organic Reactions
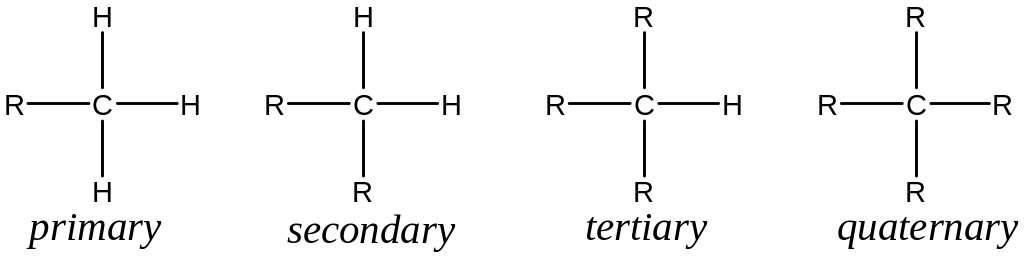
There are four possible of alkyl substitution for carbon denoted 10 (primary), 20 (secondary), 30 (tertiary) and 40 (quaternary) e.g,
1.1 Classification of organic reactions by type of chemical bonds braking
Homolysis
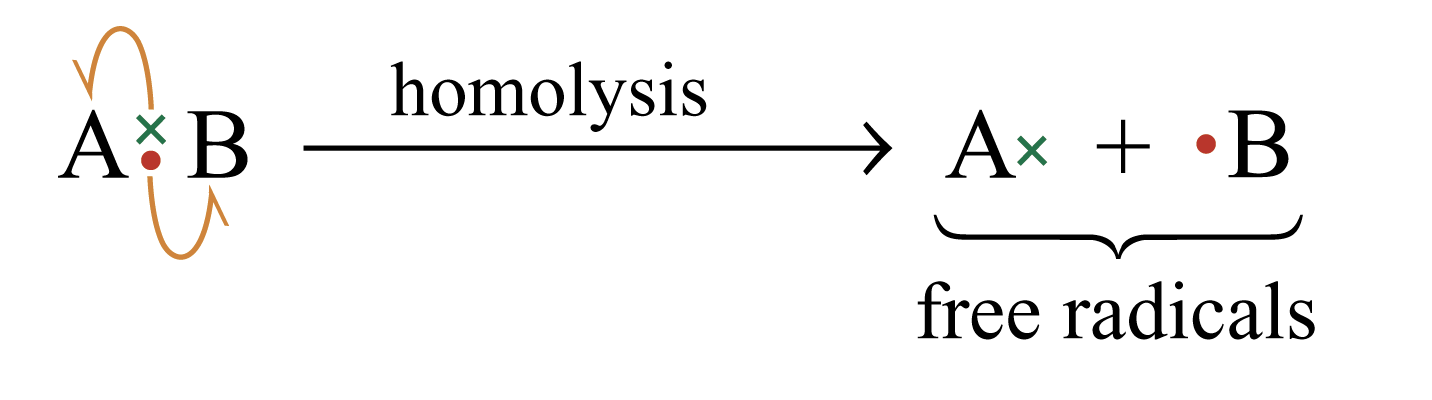
The movement of a single electron to each atoms with formation two free radicals.
Energy must be supplied in, either in form of heat or irradiation with light.
e.g. chlorine undergoes homolysis readily when heated, or when irradiated with light of a wavelength that can be absorbed by the molecule to form two chlorine radicals
![]()
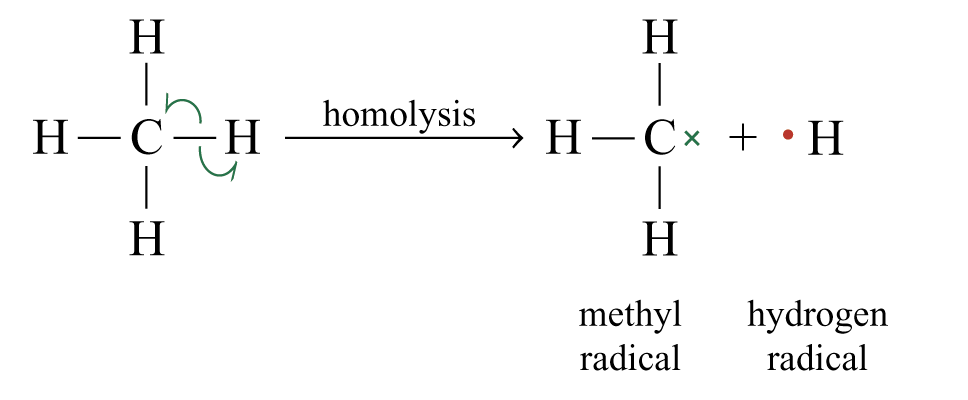
General equation of homolysis of a bond to carbon:

e.g. methane undergoes homolysis to form a methyl radical and a hydrogen radical
Free Radicals
Electrically neutral atoms or groups of atoms possessing an unpaired electron
Highly reactive because of the unstable electronic configuration
e.g.
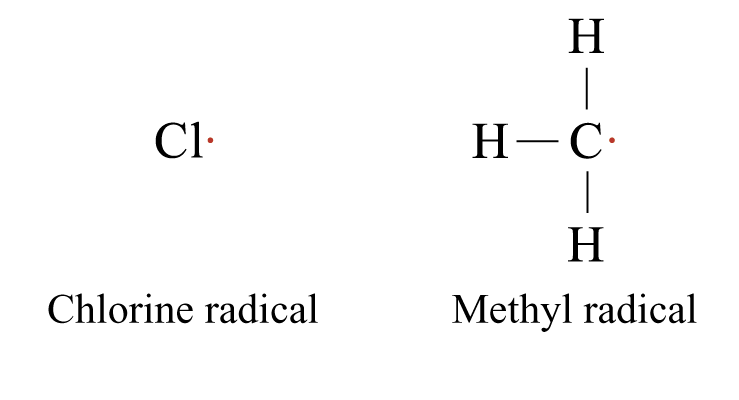
![]()
tert-butyl carboradical is the most stable because electron-donating groups exert positive inductive effects and hyperconjugation effects to stabilize carbon atom .
The greater number of alkyl groups attached to the central carbon atom, the more stable is the carboradical .

 Electron-accepting
groups exert negative inductive effects to destabilize carboradical
due to increase of electron deficit on the carbon atom.
Electron-accepting
groups exert negative inductive effects to destabilize carboradical
due to increase of electron deficit on the carbon atom.
If the carbon atom with the unpaired electron is related to the atom in a state of sp2-hybridization or with a heteroatom which has a lone pair of electrons, carboradical is more stable due to the delocalization of charge on the conjugating system (р-π-conjugation or р-р-conjugation)
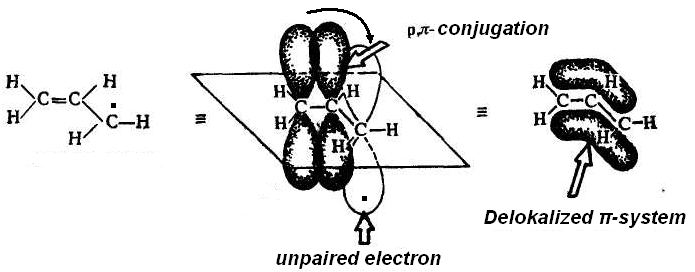
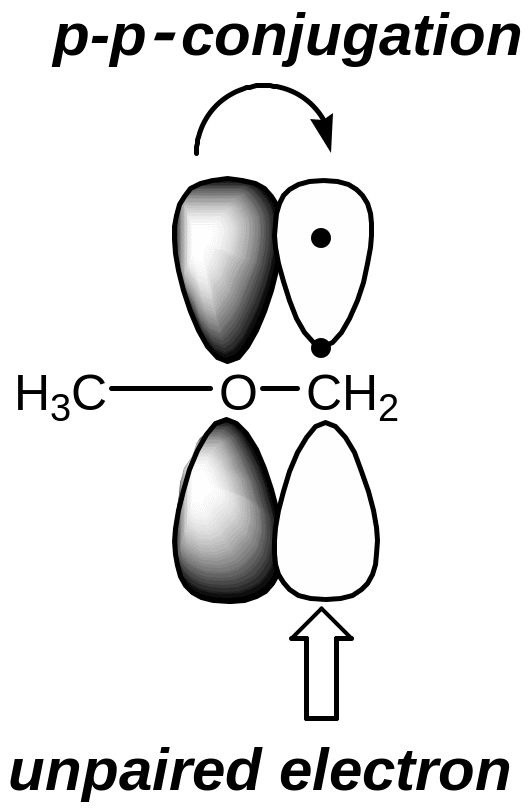
Heterolysis

The movement of a pair of electrons to one atom. Two charged fragments or ions formed.
Heterolysis of a bond requires the bond to be polarized.
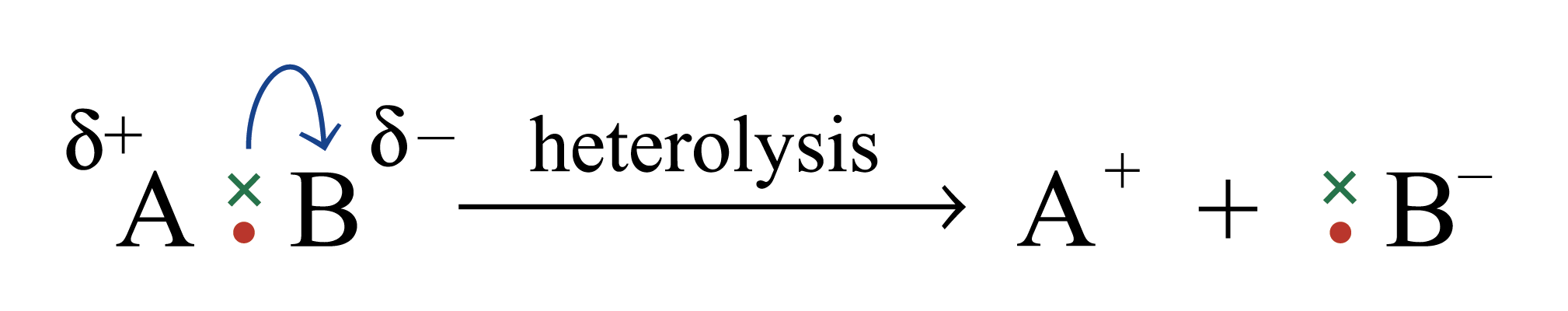
The greater the difference in electronegativity between the atoms, the greater is the polarization of the bonds.
The product of heterolysis of a bond to carbon depends on the electronegativity of the atom that is bonded to the carbon atom.
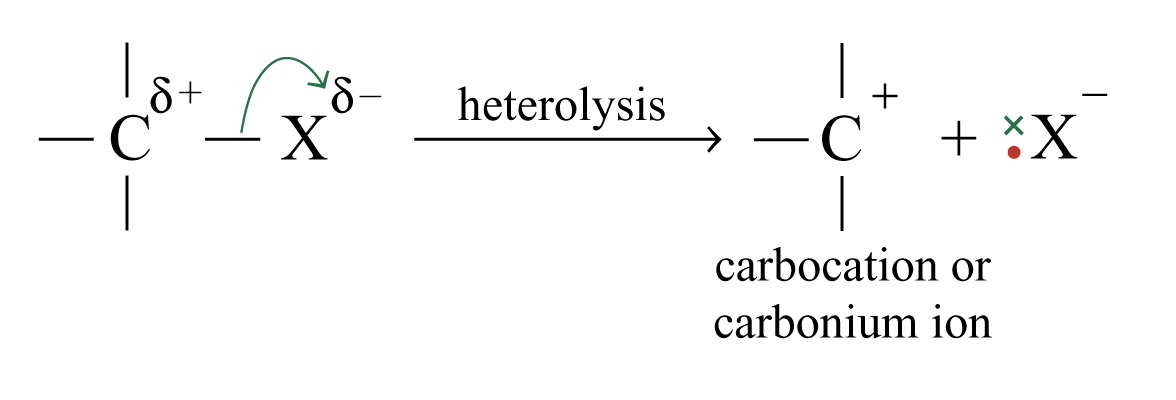
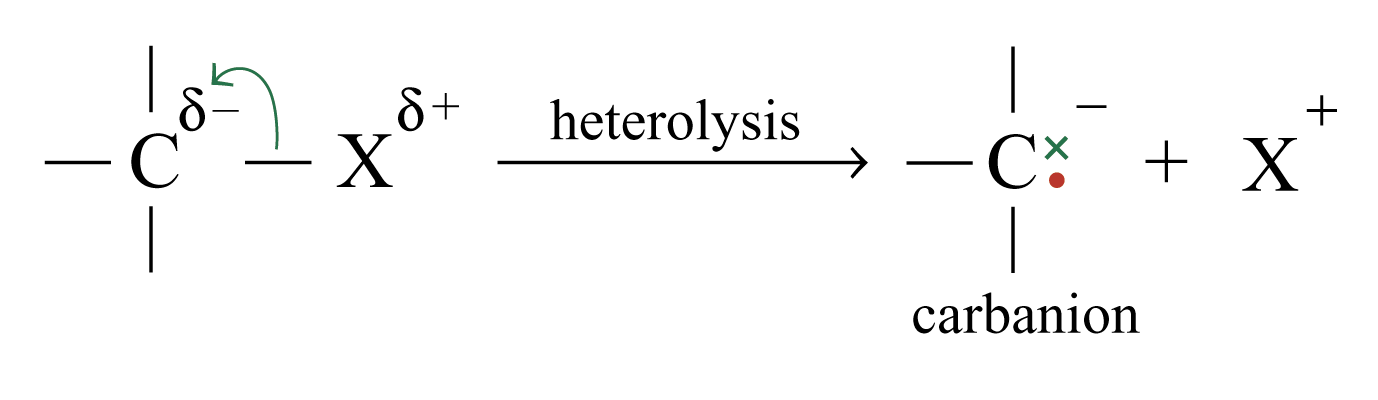
tert-butyl carbocation is the most stable because electron-donating groups exert positive inductive effects to reduce the positive charge on the carbon atom.
Like carboradical, the greater number of alkyl groups attached to the central carbon atom, the more stable is the carbocation.

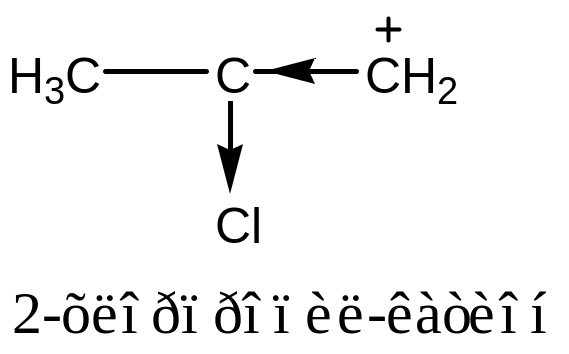 Electron-accepting
groups
exert negative inductive effects to destabilize
carbocation
due to increase of electron deficit on the carbon atom.
Electron-accepting
groups
exert negative inductive effects to destabilize
carbocation
due to increase of electron deficit on the carbon atom.
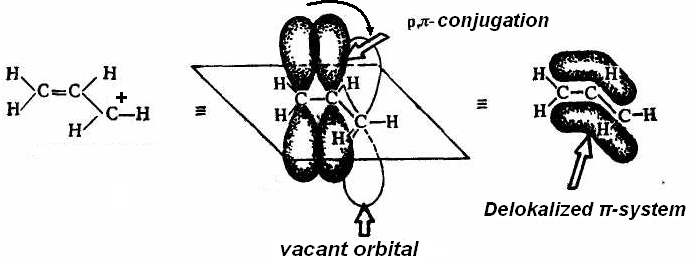
I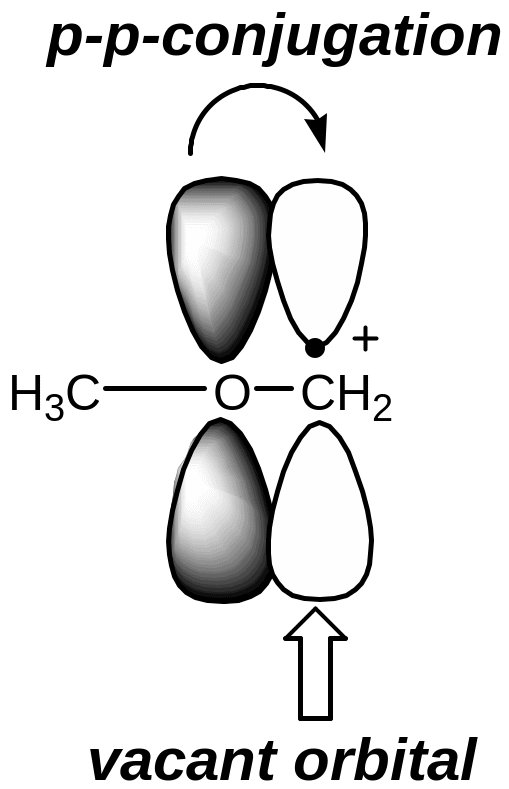 f
the carbon atom with the vacant orbital is related to the atom in a
state of sp2-hybridization
or with a heteroatom which has a lone pair of electrons, carbocation
is more stable due to the delocalization of charge on the conjugating
system (р-π-conjugation
or р-р-conjugation)
f
the carbon atom with the vacant orbital is related to the atom in a
state of sp2-hybridization
or with a heteroatom which has a lone pair of electrons, carbocation
is more stable due to the delocalization of charge on the conjugating
system (р-π-conjugation
or р-р-conjugation)
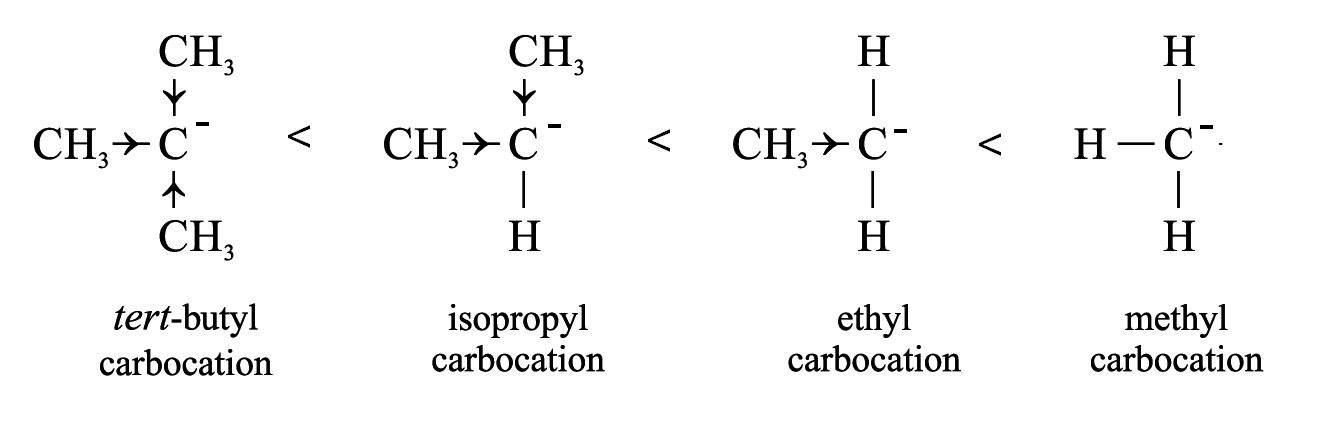
tert-butyl carboanion is the least stable because electron-donating groups exert positive inductive effects to increase the negative charge on the carbon atom.
Unlike carbocation and carboradical, the greater the number of alkyl groups attached to the central carbon atom, the less stable is the carboanion.
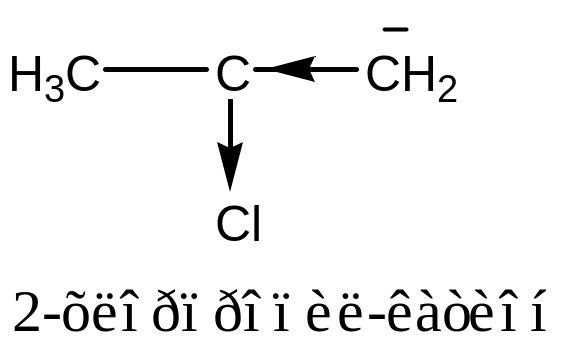 Electron-accepting
groups exert negative inductive effects to stabilize carboanion due
to decrease of electron over on the carbon atom.
Electron-accepting
groups exert negative inductive effects to stabilize carboanion due
to decrease of electron over on the carbon atom.
