
- •Types of Organic Reactions
- •Isomerism
- •Types of Organic Reactions
- •1.1 Classification of organic reactions by type of chemical bonds braking
- •Classification of organic reactions by nature of reagent.
- •Classification by the type of organic reactions
- •Classification by number of molecules, which take part in the slowest step of organic reaction (by order reaction)
- •Isomerism
- •2.1. Structural Isomerism
- •Stereoisomerism
Stereoisomerism
Formulas for imaging of stereo isomers
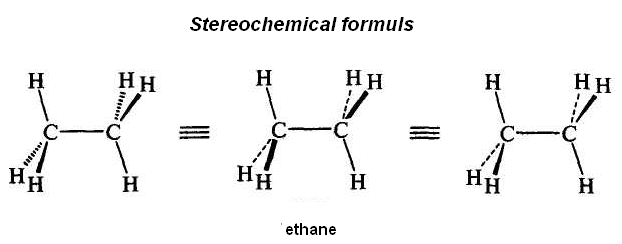



Conformational Isomerism
Conformational isomers are stereoisomers that have different arrangements of their atoms in space due to free rotation about a covalent σ-bond.
e.g.

C
 onformational
isomers
interconvert into each other without breaking of chemical bonds.
Unlike structural isomers, different conformers can’t usually be
isolated, because they
interconvert
too
rapidly. This kind of isomers can be fined by physical and chemical
methods only.
onformational
isomers
interconvert into each other without breaking of chemical bonds.
Unlike structural isomers, different conformers can’t usually be
isolated, because they
interconvert
too
rapidly. This kind of isomers can be fined by physical and chemical
methods only.
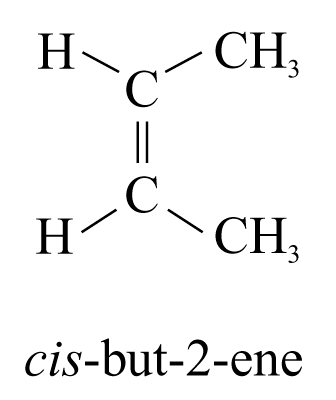
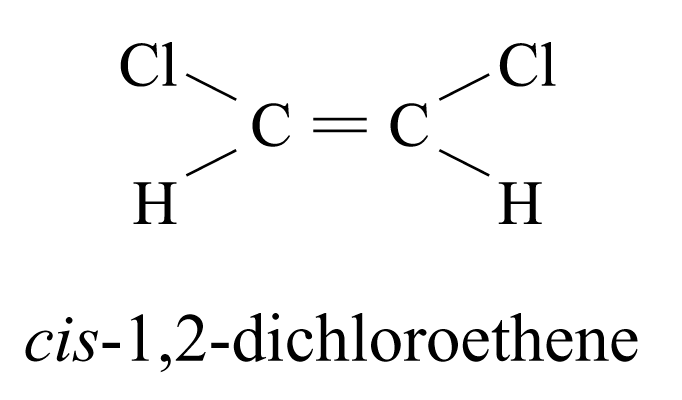
Geometrical Isomerism
Geometrical isomers are stereoisomers that have different arrangements of their atoms in space due to restricted rotation about a covalent bond.
R otation
of a carbon atom of a C = C bond through an angle of 90°
causes the breaking
of the p
bond.
otation
of a carbon atom of a C = C bond through an angle of 90°
causes the breaking
of the p
bond.



Geometrical isomers have different physical and chemical properties.
Physical properties |
cis-But-2-ene |
trans-But-2-ene |
Melting point (°C) |
–139 |
–106 |
Boiling point (°C) |
4 |
1 |
trans-But-2-ene has higher melting point
Þ more regular and symmetrical structure
Þ molecules pack more compactly in crystal lattice
Þ difficult to break the lattice
Þ higher melting point
cis-But-2-ene has higher boiling point because it has net dipole moment

Þ molecules are held together by dipole-dipole interactions
Þ trans-isomer has no net dipole moment, their molecules are held by instantaneous dipole-induced dipole interactions
Þ dipole-dipole interactions are stronger
Þ cis-but-2-ene has higher boiling point
Another example:
cis-butenedioic acid and trans-butenedioic acid
Physical properties |
cis-Butenedioic acid |
trans-Butenedioic acid |
Melting point (°C) |
130 |
286 |
Solubility in water (gram of solution per 100 g of water at 25 °C) |
78.8 |
0.7 |
trans-butenedioic acid has higher melting point and form more extensive intermolecular hydrogen bonding.
While the cis-isomer forms intramolecular hydrogen bonding Þ less extensive intermolecular hydrogen bonding Þ molecules are less strongly held Þ lower melting point


Although trans-butenedioic acid can form more extensive hydrogen bonds with water molecules, cis-butenedioic acid is more soluble in water than the trans-isomer because of the greater dipole moment.

cis- and trans-butenedioic acids have different chemical properties

Enantiomerism
Enantiomerism occurs in those compounds whose molecules are chiral.
A chiral molecule is one that is not superimposable with its mirror image.
The chiral molecule and its mirror image are enantiomers.




sp3-hybridized carbon atom with two or more identical groups attached is achiral and contains a plane of symmetry
sp3-hybridized carbon atom with four different groups attached is chiral and do not contain a plane of symmetry
e.g. butan-2-ol

Properties of Enantiomers: Optical Activity
Enantiomers have identical physical properties
Physical properties |
(+) Butan-2-ol |
(–) Butan-2-ol |
Boiling point (°C) |
99.5 |
99.5 |
Density (g cm–3 at 20°C) |
0.808 |
0.808 |
Refractive index (at 20°C) |
1.397 |
1.397 |
One easily observable difference of a pair of enantiomers is their properties towards plane-polarized light. When a beam of plane-polarized light passes through a solution of an enantionmer, the plane of polarization rotates.
Two enantiomers that are mirror images of one another cause exactly the same extent of rotation, but in opposite directions, clockwise in one and anticlockwise in the other.
Enantiomers are also called optical isomers and said to be optically active.
Plane-polarized Light
Light is an electromagnetic radiation.

Polarimeter is a device used for measuring the effect of optically active compounds on plane-polarized light.

If the tube of polarimeter is empty, or if an optically inactive substance is present, the axes of the plane-polarized light and the analyzer will be exactly parallel.
If the tube contains an optically active substance, the plane-polarized light will rotate as it passes through the tube.
If the analyzer is rotated in a clockwise direction, the rotation is said to be positive (+).
If the rotation is anticlockwise, the rotation is said to be negative (–).
Molecule whith one chiral carbon atom exists in forms of two stereoisomers.
A compound with n chiral atoms can have a maximum of 2n stereoisomers.
N = 2n, where N – number of optical isomers ; n – number of chiral atoms
e.g. N = 22 = 4

Molecule having two chiral centers with identical substituent exists in three stereoisomeric forms. Because one isomer has a symmetry plane and is achiral.
Compounds that are achiral, yet contain stereogenic centers, are called meso compounds (meso forms).
e .g.
.g.
Tartaric acid exists in three stereoisomeric forms: two enantiomers and one meso form.
Racemic mixtures, or racemates, are 50:50 mixtures of (+) and (-) enantiomers. Racemic mixtures and individual diastereomers differ in their phisical properties, such as solubility, melting point, and boiling point.
Most reactions give chiral prodacts. If the reactant are optically inactive, the products are also optically inactive – either meso or racemic. If one ore both of the reactant is optically active, the product can also be optically active.
Questions and problems
Give the symbol for the first-order nucleophile addition reaction а) ЕS1; b) NA1; c) AN1; d) AE1 ; e) SN2; f) E; g) SE1.
What kind of reaction is the following one?
 а)
rearrangement;
b) condensation;
c) addition;
d) substitution;
а)
rearrangement;
b) condensation;
c) addition;
d) substitution;
Give the symbol for the following reaction
 а)
ЕS1;
b) NA1;
c) AN1;
d) AE1
; e) SN2;
f) E; g) SE1.
а)
ЕS1;
b) NA1;
c) AN1;
d) AE1
; e) SN2;
f) E; g) SE1.Dispose the carbocations as their stability grows: 1)
 ,
2)
,
2)
 ,
3)
,
3)
 .
Explain.
а) 1,2,3; b) 3,1,2;
c) 2,3,1; d) 1,3,2 ; e) 2,1,3; f) 3,2,1.
.
Explain.
а) 1,2,3; b) 3,1,2;
c) 2,3,1; d) 1,3,2 ; e) 2,1,3; f) 3,2,1.What kind of isomerism is there in the following pair of compounds
 а) chain
isomerism;
b) position
isomerism;
c) metamerism;
d) tautomerism;
e) functional
group
isomerism;
f) enantiomerism;
g) geometrical
isomerism;
h) conformational
isomerism.
а) chain
isomerism;
b) position
isomerism;
c) metamerism;
d) tautomerism;
e) functional
group
isomerism;
f) enantiomerism;
g) geometrical
isomerism;
h) conformational
isomerism.What kind of formulas was used in the previous question? а) full structural; b) short structural; c) simplified structural; d) sterechemical; e) perspective; f) Newman formula; g) Fischer formula.
What kind of isomers has the same empirical formulas but different functional groups? а) chain isomerism; b) position isomerism; c) metamerism; d) tautomerism; e) conformational isomerism; f) enantiomerism; g) geometrical isomerism.
 How
many
optical
isomers
has
the
following
compound?
In
the
following
compound
show
chiral
atoms
as
*. а) 2; b) 3; c) 4; d) 9; e) 8; f) compound
has
no
optical
isomers.
How
many
optical
isomers
has
the
following
compound?
In
the
following
compound
show
chiral
atoms
as
*. а) 2; b) 3; c) 4; d) 9; e) 8; f) compound
has
no
optical
isomers.
