
- •1 Introduction
- •2 Nomenclature of Halogeno-compounds
- •3 Physical Properties of Halogeno-compounds
- •4 Preparation of Halogeno-compounds
- •5 Reactions of Halogeno-compounds
- •6 Nucleophilic Substitution Reactions
- •1. Sn2 reactions
- •2. Sn1 reactions
- •1. Experiment 1 : Comparison of the rates of hydrolysis of 1-chlorobutane, 1-bromobutane and 1-iodobutane
- •2. Experiment 2: Comparison of the rates of hydrolysis of primary, secondary and tertiary haloalkanes and halobenzene
- •7 Elimination Reactions
- •8 Uses of Halogeno-compounds
1. Experiment 1 : Comparison of the rates of hydrolysis of 1-chlorobutane, 1-bromobutane and 1-iodobutane
(a) Objective
To study the effect of the nature of the halogen leaving group on the rate of hydrolysis of haloalkanes
(b) Procedure
Put 2 cm3 of ethanol and 1 cm3 of 0.1 M aqueous silver nitrate into each of three test tubes
Place them in a water bath at 60°C
After 5 mins, add 5 drops of 1-chlorobutane the test tube A, 5 drops of 1-bromobutane to B and 5 drops of 1-iodobutane to C
Shake each test tube and observe for 10 mins

(c) Result and Observation
A precipitate of silver halide is formed in each of the three test tubes



(d) Discussion
Water molecule is the nucleophile of the reaction
Haloalkanes react with water by nucleophilic substitutions
The halide ion departs as the leaving group
The ease of leaving of halide ions decreases: I– > Br– > Cl–
The order of precipitates appeared tends to follow the order of ease of leaving of the halide ions, which subsequently form precipitates with Ag+ ions from AgNO3
Ag+(aq) + X–(aq) ®¾ AgX(s)
2. Experiment 2: Comparison of the rates of hydrolysis of primary, secondary and tertiary haloalkanes and halobenzene
(a) Objective
To study the effect of the structure of haloalkanes on the rate of hydrolysis of them and to compare the rates of hydrolysis of haloalkanes and halobenzene
(b) Procedure
Put 2 cm3 of ethanol and 1 cm3 of 0.1 M aqueous silver nitrate into each of four test tubes
Add 5 drops of 1-chlorobutane the test tube D, 5 drops of 2-chlorobutane to E, 5 drops of 2-chloro-2-methylpropane to F and 5 drops of chlorobenzene to G
Shake each test tube well and observe for 10 mins

(c) Result and Observation
Except test tube G, a white precipitate of silver chloride was formed in each of test tubes D, E and F.




(d) Discussion
The halogen-compounds used in the experiment are of different classes:
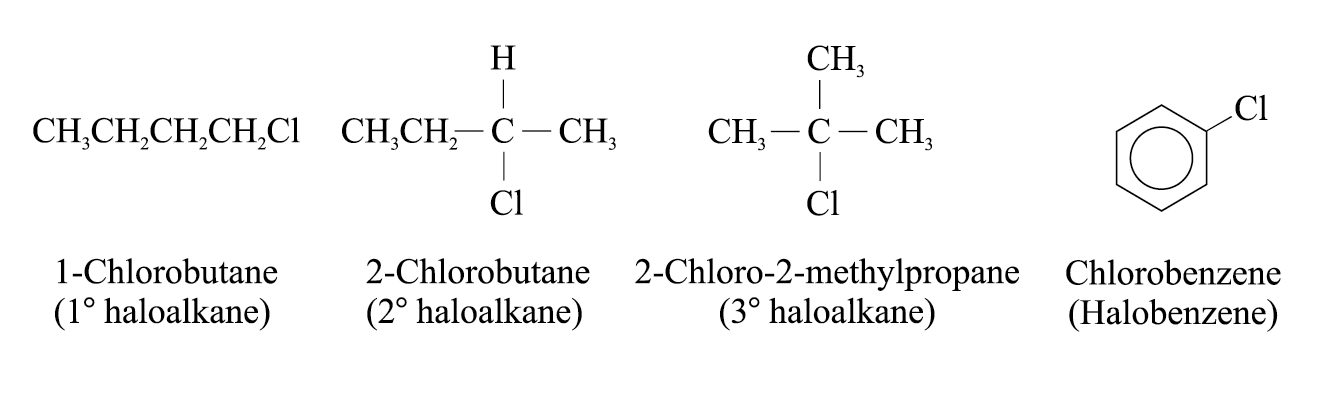
The rate of formation of the white precipitate of silver chloride decreases in the order:
2-chloro-2-methylpropane > 2-chlorobutane » 1-chlorobutane >> chlorobenzene
The rate of hydrolysis of halogeno-compounds is related to the structure of the substrate around the carbon which is being attacked
The experimental condition favours SN1 reactions. Tertiary haloalkane reacts at the fastest rate while primary haloalkane proceeds at a slower rate
Chlorobenzene can be hydrolyzed to phenol under severe conditions (cannot be carried out in school laboratory)

Unreactivity of Halobenzene
Halobenzenes are comparatively unreactive to nucleophilic substitution reactions. The p orbital on the carbon atom of the benzene ring and that on the halogen atom overlap side-by-side to form a delocalized p bonding system

Delocalization of p electrons throughout the ring and halogen atom. The C–X bond has partial double bond character Þ stronger than that of haloalkane Þ larger amount of energy is required to break the bond Þ substitution reactions become more difficult to occur.
Delocalization of p electrons makes the polarity of C–X bond ¯ Þ electropositive carbon center is less susceptible to nucleophilic attack.
Delocalized electrons repel any approaching nucleophiles Þ unreactive towards SN2 reactions
Benzene cations are highly unstable because of loss of aromaticity Þ unreactive towards SN1 reactions
Reaction with Potassium Cyanide
A nitrile is formed when a haloalkane is heated under reflux with an aqueous alcoholic solution of potassium cyanide


Cyanide ion (CN–) acts as a nucleophile
Halobenzenes do not react with potassium cyanide
The reaction is very useful because the nitrile can be hydrolyzed to carboxylic acids which can be reduced to alcohols
![]()
![]()
A useful way of introducing a carbon atom into an organic molecule, so that the length of the carbon chain can be increased
Reaction with Ammonia
When a haloalkane is heated with an aqueous alcoholic solution of ammonia under a high pressure, an amine is formed
![]()

Ammonia is a nucleophile because the presence of a lone pair of electrons on the nitrogen atom
As the lone pair electrons on nitrogen atom in ethylamine are still available, the ethylamine will compete with ammonia as the nucleophile.
A series of further substitutions take place
A mixture of products is formed


The reaction stops at the formation of a quaternary ammonium salt
The competing reactions can be minimized by using an excess of ammonia
