
- •1 Introduction
- •2 Nomenclature of Halogeno-compounds
- •3 Physical Properties of Halogeno-compounds
- •4 Preparation of Halogeno-compounds
- •5 Reactions of Halogeno-compounds
- •6 Nucleophilic Substitution Reactions
- •1. Sn2 reactions
- •2. Sn1 reactions
- •1. Experiment 1 : Comparison of the rates of hydrolysis of 1-chlorobutane, 1-bromobutane and 1-iodobutane
- •2. Experiment 2: Comparison of the rates of hydrolysis of primary, secondary and tertiary haloalkanes and halobenzene
- •7 Elimination Reactions
- •8 Uses of Halogeno-compounds
Lecture 8
Halogeno-compounds
Introduction
Nomenclature of Halogeno-compounds
Physical Properties of Halogeno-compounds
Preparation of Halogeno-compounds
Reactions of Halogeno-compounds
Nucleophilic Substitution Reactions
Elimination Reactions
Uses of Halogeno-compounds
1 Introduction
Haloalkanes are organic compounds having one or more halogen atoms replacing hydrogen atoms in alkanes
Haloalkanes are classified into primary, secondary and tertiary, based on the number of alkyl groups attached to the carbon atom which is bonded to the halogen atom
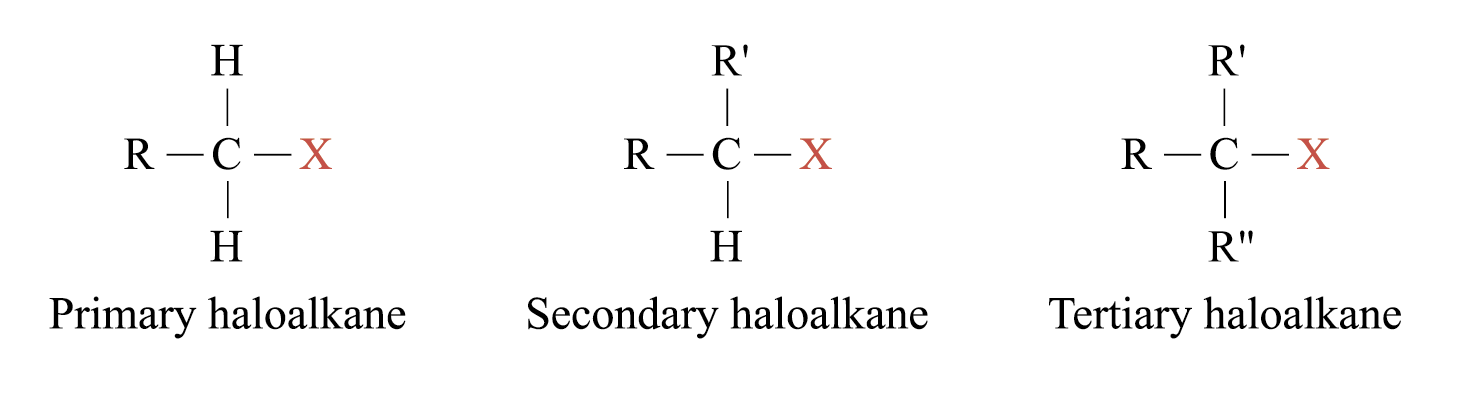
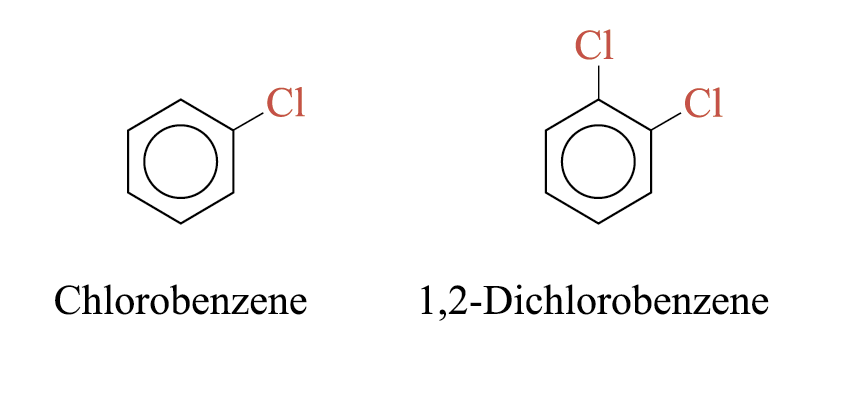
Halobenzenes are organic compounds in which the halogen atom is directly attached to a benzene ring e.g.
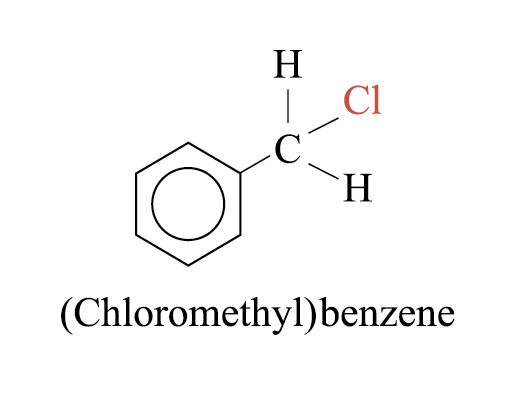
(Cloromethyl)benzene is not a halobenzene, because the chlorine atom is not directly attached to the benzene ring
2 Nomenclature of Halogeno-compounds
Naming haloalkanes are similar to those for naming alkanes
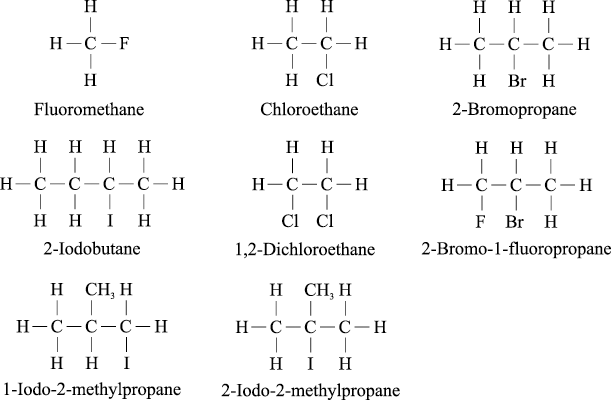
The halogens are written as prefixes: fluoro- (F), chloro- (Cl), bromo- (Br) and iodo- (I)
e.g.
When the parent chain has both a halogen and an alkyl substituent, the chain is numbered from the end nearer the first substituent regardless of what substituents are
e.g.
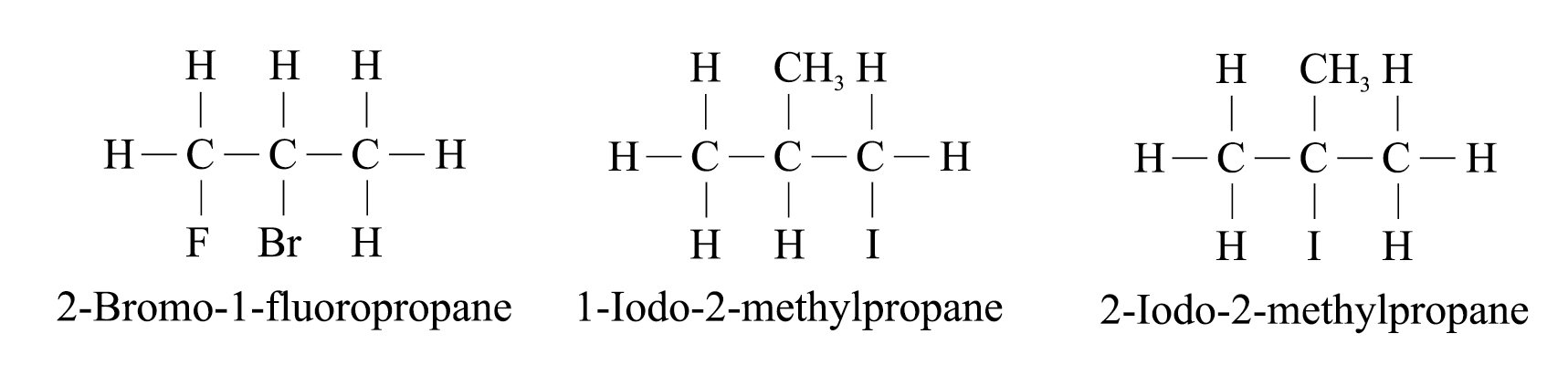
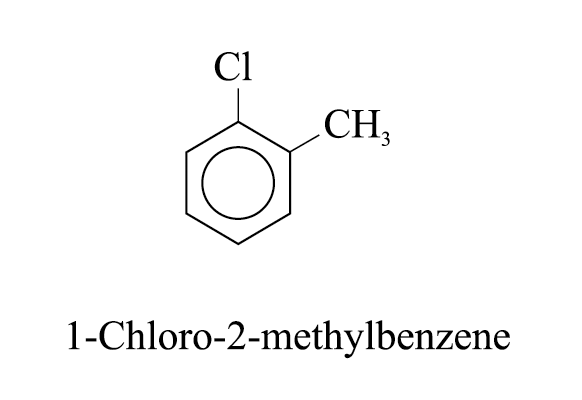
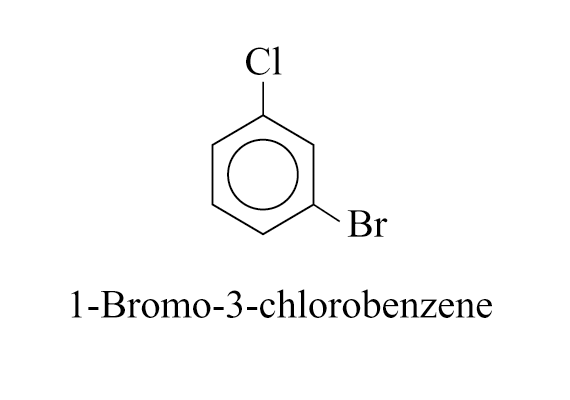
In case of halobenzenes, the benzene ring is numbered so as to give the lowest possible numbers to the substituents
e.g.

3 Physical Properties of Halogeno-compounds
Boiling Point and Melting Point
Haloalkanes have higher b.p. and m.p. than alkanes. Dipole-dipole interactions are present between haloalkane molecules
m.p. and b.p. increase in the order: RCH2F < RCH2Cl < RCH2Br < RCH2I Larger, more polarizable halogen atoms increase the dipole-dipole interactions between the molecules
No. of carbon Þ m.p. and b.p.
Density
Relative molecular mass Þ density ¯. Closer packing of the smaller molecules in the liquid phase
Bromo and iodoalkanes are all denser than water at 20°C
Solubility
Although C — X bond is polar, it is not polar enough to have a significant effect on the solubility of haloalkanes and halobenzenes Þ Immiscible with water Þ Soluble in organic solvents
4 Preparation of Halogeno-compounds
Preparation of Haloalkanes
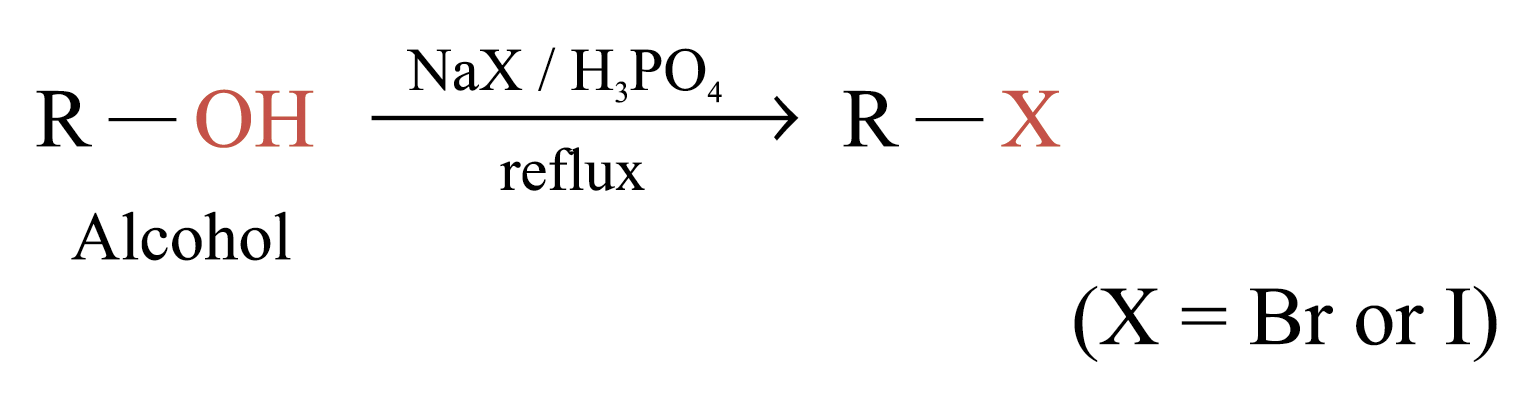
Substitution of Alcohols
Prepared by substituting –OH group of alcohols with halogen atoms
Common reagents used: HCl, HBr, HI, PCl3 or PBr3
The ease of substitution of alcohols: 3° alcohol > 2° alcohol > 1° alcohol > CH3OH
This is related to the stability of the reaction intermediate (i.e. stability of carbocations)
Reaction with Hydrogen Halides
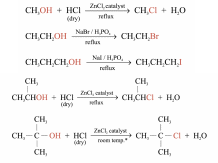
Dry HCl is bubbled through alcohols in the presence of ZnCl2 catalyst
![]()
For the preparation of bromo- and iodoalkanes, no catalyst is required
The reactivity of hydrogen halides: HI > HBr > HCl
e.g.
Reaction with Phosphorus Halides
Haloalkanes can be prepared from the vigorous reaction between cold alcohols and phosphorus(III) halides

Addition of Alkenes and Alkynes
Addition of halogens or hydrogen halides to an alkene or alkyne can form a haloalkane
e.g.
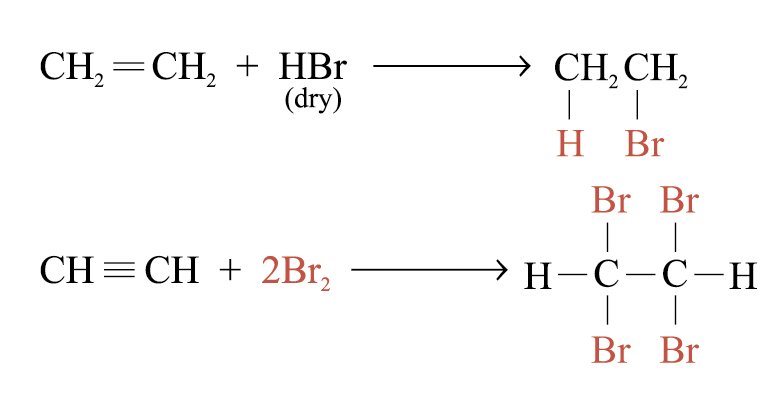
Preparation of Halobenzenes
Halogenation of Benzene
Benzene reacts readily with chlorine and bromine in the presence of catalysts (e.g. FeCl3, FeBr3, AlCl3)
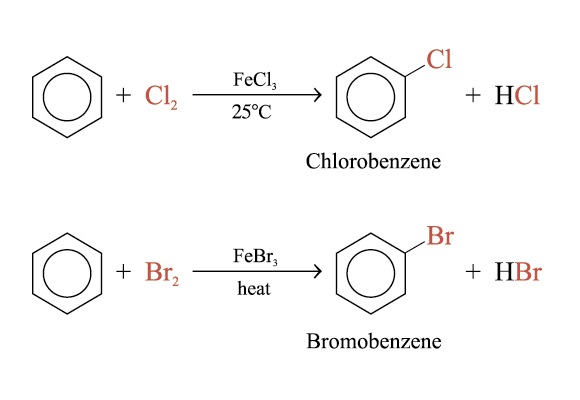
From Benzenediazonium Salts
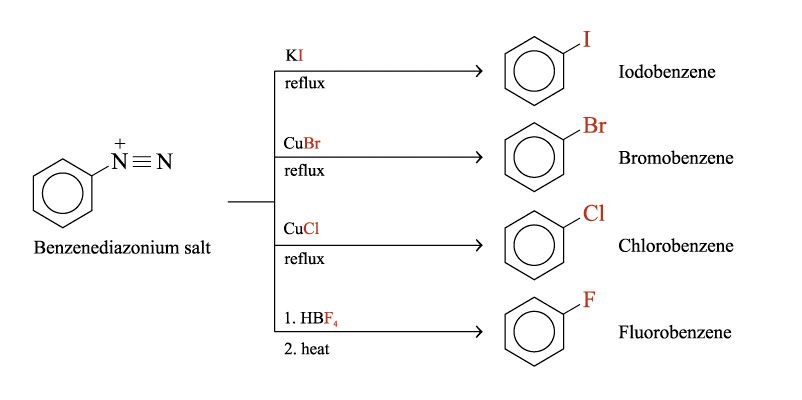
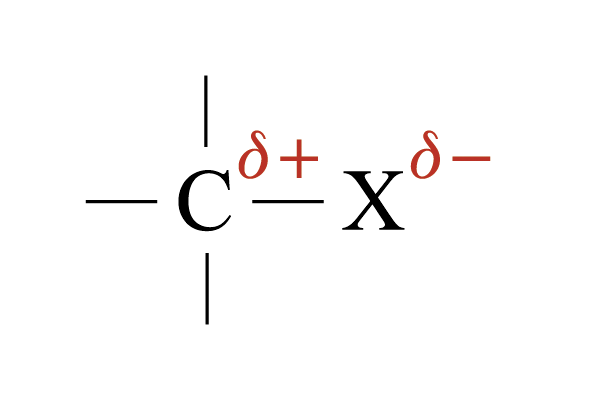
5 Reactions of Halogeno-compounds
Carbon-halogen bond is polar
Carbon atom bears a partial positive charge
Halogen atom bears a partial negative charge
Characteristic reaction: Nucleophilic substitution reaction
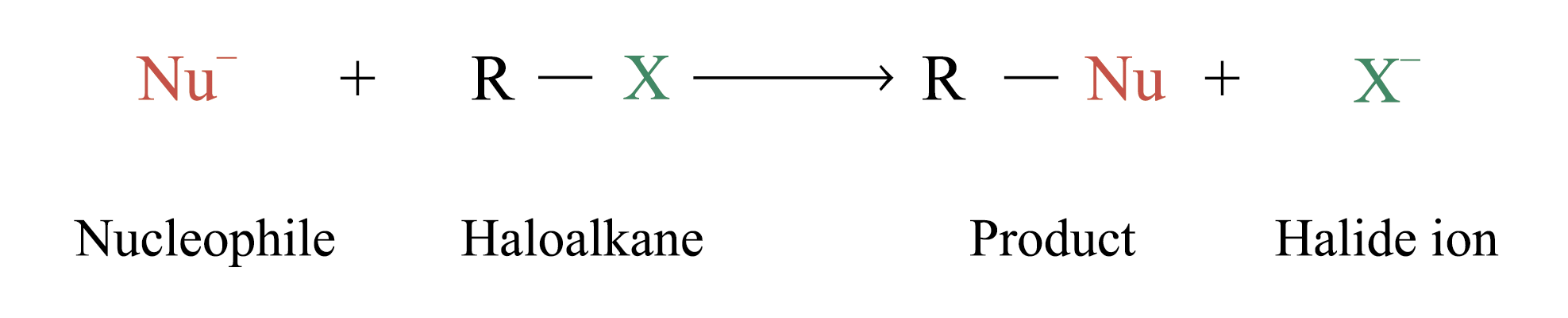
Alcohols, ethers, esters, nitriles and amines can be formed by substituting – OH, – OR, RCOO –, – CN and – NH2 groups respectively
Another characteristic reaction: Elimination reaction
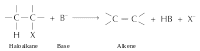
Bases and nucleophiles are the same kind of reagents
Nucleophilic substitution and elimination reactions always occur together and compete each other
