
Dale_Molecular Genetics of Bacteria 4th ed
.pdfREGULATION OF GENE EXPRESSION |
87 |
NH2-terminal domain binds arabinose and mediates dimerization, while the COOH-terminal domain contains the regions that bind to the DNA. When arabinose binds to the NH2-terminal domain, it alters the way that the dimer forms and hence its ability to contact different sites on the DNA. As a consequence, AraC no longer binds to the upstream site (and therefore the DNA is no longer looped), but binds instead to two adjacent sites close to the promoter, resulting in activation of the promoter. Thus in the absence of arabinose AraC is a negative regulator while in the presence of the substrate it acts as a positive regulator.
Other transcriptional regulators
As mentioned earlier, the lac repressor belongs to the class of regulators containing helix-turn-helix motifs. This is the most common DNA-binding protein motif in prokaryotes. Many other transcriptional regulators with very diverse roles also contain this motif, which can be used to identify putative regulator genes in genome sequence data. In E. coli alone there are 314 transcriptional regulators with helix-turn-helix motifs. Generally, in this class of regulator the protein is guided to specific DNA sequences by the helix-turn-helix motif which is linked to a larger regulatory domain which regulates the DNA binding activity. In the case of CRP (see above), the signal molecule cAMP binds to the regulatory domain and allosterically controls the DNA binding activity of the regulator.
Amongst other examples, a protein known as FNR, which has a similar structure to CRP, controls the transcription of a wide range of genes whose functions are necessary for growth under oxygen limitation. Regulatory proteins such as FNR (and CRP) that influence the activity of a large number of genes are known as global regulators. The DNA-binding activity of FNR is regulated by oxygen availability with a cluster of iron and sulphur molecules [4Fe-4S]2þ forming the actual oxygen sensor. OxyR is also a member of this class of regulators, and globally regulates the expression of genes in response to hydrogen peroxide stress. In this protein, the sensor for the oxidizing agent is a motif containing two cysteine residues. Oxidation of these residues by hydrogen peroxide causes the formation of an intramolecular disulphide bond and results in a conformational change which allows OxyR to bind to its target genes. Alternative mechanisms for global regulation are considered later in this chapter.
3.2.4 Attenuation: trp operon
The lac and ara operons are both examples of inducible systems, i.e. they are switched on in the presence of the relevant substrate. On the other hand, when dealing with biosynthetic pathways, such as the production of amino acids, the
88 |
MOLECULAR GENETICS OF BACTERIA |
converse applies: expression of the genes concerned should be switched off if the end-product is present. This can also be achieved by the use of regulatory proteins, with only a minor modification of the model. For example, the trp operon, coding for the genes required for synthesis of tryptophan, is regulated (in E. coli) by a protein, TrpR, which is unable by itself to interact with the operator region of the trp operon; it requires binding of tryptophan to form the active repressor.
The trp operon is also regulated by a completely different process, known as attenuation, which involves transcriptional termination as described earlier in this chapter. The operon (Figure 3.19) contains a sequence (of 162 bases), known as the leader sequence, between the transcription start point and the start of the first structural gene. The leader sequence has several mutually complementary regions that can form alternative stem–loop structures under different conditions. Of these alternative secondary structures, only the 3:4 stem–loop structure will actually cause termination.
The leader sequence contains a region that can be translated into a short peptide (Figure 3.20). As soon as a long enough portion of the mRNA is made, ribosomes will bind to it and start translation. The sequence however contains two tryptophan codons, thus requiring the presence of tryptophan within the cell for incorporation into the peptide. In the absence of tryptophan, the ribosomes will therefore stall at that point. This is within the sequence designated 1; the presence of the ribosomes on this sequence will block its pairing with sequence 2, which is thus free to form a stem–loop structure with region 3 as soon as the latter region is produced. This structure (2:3) is not a terminator and does not block further transcription, but it sequesters region 3 and prevents it pairing to region 4 (see Figure 3.21b). This therefore prevents the formation of the 3:4 stem–loop structure which would otherwise terminate transcription. Thus in the absence of tryptophan, expression of the trp operon is permitted.
|
|
|
|
Terminator |
p/o Leader /attenuator |
trpE |
trpD |
trpC |
trpA |
1 |
2 |
|
3 |
4 |
Inverted repeats
Figure 3.19 Structure of the trp operon

|
REGULATION OF GENE EXPRESSION |
89 |
||||||||||||
|
GGGAAAAAUGCACUUGAA 5' |
|
|
|
|
|
|
|
|
|
|
|
|
|
U |
Leader peptide |
|
|
|
|
|
|
|
|
|
|
|
|
|
A |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
U |
M K A I F V L K G W W R T S * |
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
||||||
|
CGACAAUGAAAGCAAUUUUCGUACUGAAAGGUUGGUGGCGCACUUCCUGA |
|
|
A |
||||||||||
|
|
|
|
1 |
|
|
|
|||||||
|
|
|
2 |
|
|
|
A |
|||||||
|
|
G CG U |
A C CA C U U AU G U G AC G G |
G C |
||||||||||
|
U |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A |
|
3 |
|
|
|
|
|
|
|
||||
|
|
A |
|
|
|
|
|
|
|
|||||
|
|
AGCAAUCAG |
|
|
|
|
|
|
|
|
|
|||
|
|
|
AUACCCAGCCCGCC |
U |
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
A |
||
|
|
|
4 |
|
|
A |
||||||||
|
|
|
|
|
U |
|||||||||
|
|
|
|
|
A G UUUUUUUU |
CGGGCGAG |
||||||||
|
|
|
|
|
A |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C |
|
|
|
|
|
||||
|
|
|
|
|
A AAAUUAGAGAAUAACAAUGCAAACA |
|||||||||
|
|
|
|
|
|
|
|
|
|
|
M Q T |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TrpE |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
||
Figure 3.20 Structure of the leader–attenuator region of the trp operon mRNA. The boxed sequences 1–4 are the regions capable of forming alternative stem–loop structures
In the presence of sufficient tryptophan, the ribosomes can proceed unimpaired through the leader sequence until they reach the stop codon which is adjacent to the start of region 2. Although the ribosomes would eventually dissociate from the mRNA, they occupy this position for long enough to ensure that region 2 is prevented from forming a stem–loop structure. If 2:3 does not form rapidly, then as soon as region 4 is made, the more stable 3:4 structure will form (Figure 3.21c). Since 3:4 is a genuine terminator, transcription will stop. The presence of tryptophan therefore prevents expression of the trp operon. Similarly, if protein synthesis is prevented altogether, then 1 will pair with 2 and 3 with 4, also leading to repression of the operon (Figure 3.21a).
Similar attenuation methods control a number of other operons concerned with the biosynthesis of amino acids. In each case, the operon has a leader sequence that is translated into a peptide containing multiple codons for the amino acid concerned. For example, the his operon in E. coli has a leader sequence that codes for a run of seven histidines, while the pheA attenuator region has seven phenylalanine codons. Stalling of the ribosome at these codons prevents the formation of the terminator structure.
In B. subtilis, the trp operon is also controlled by attenuation at the transcriptional level (Figure 3.22), but the mechanism is an intriguing variation of that described above for E. coli. In this case stem–loop structures can occur in regions A:B or C:D of the leader sequence of the trp mRNA but only the latter structure will actually cause termination. In the absence of tryptophan the structure A:B forms; because of the overlap between B and C, this prevents the formation of C:D so transcription of the operon occurs. The key differences between the E. coli and B. subtilis system are the presence of 11 repeated DNA sequences (GAG or UAG) in the leader mRNA and an RNA binding protein called TRAP (trp RNAbinding Attenuation Protein) which, in the presence of tryptophan, is able to bind

90 |
MOLECULAR GENETICS OF BACTERIA |
(a) Absence of protein synthesis
Termination
mRNA
5
1 |
2 |
3 |
4 Stem-loop causes |
|
|
|
transcriptional |
|
|
|
termination |
(b) Protein synthesis with limiting tryptophan |
|
|
|||||||
1 |
|
|
|
|
|
|
4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ribosome stalled |
2 |
|
|
|
3 |
Formation of 3:4 terminator is |
|||
|
|
|
|||||||
at trp codons, |
|
|
|
|
|
prevented. Transcription |
|||
blocks formation |
|
|
|
|
|
continues |
|||
of 1:2 structure |
|
|
|
|
|
|
|
||
(c) Protein synthesis with sufficient tryptophan |
|
|
|
|
|
|
||||||
1 |
2 |
|
|
|
|
|
Termination |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ribosome stalled |
3 |
|
|
|
4 |
|
|||
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
||||||
|
|
|
at stop codon, |
|
|
|
|
Stem-loop causes |
||||
|
|
|
blocks 1:2 and |
|
|
|
|
transcriptional |
||||
|
|
|
2:3 structures |
|
|
|
|
|||||
|
|
|
|
|
|
|
termination |
|||||
|
|
|
|
|
|
|
|
|
|
|
||
Figure 3.21 Attenuation control of the trp operon. (a) In the absence of protein synthesis, the terminator stem–loop 3:4 is able to form, and the operon is not transcribed.
(b) If protein synthesis occurs in the presence of limiting amounts of tryptophan, ribosomes will stall at the tryptophan codons in the leader region, blocking formation of the 1:2 stem–loop. When the RNA polymerase transcribes region 3, it will pair with region 2. The 2:3 structure is not a terminator, but it sequesters region 3, thus preventing formation of the 3:4 terminator and allowing transcription of the operon. (c) In the presence of sufficient tryptophan, the ribosomes will proceed as far as the stop codon, thus blocking both regions 1 and 2. This allows the 3:4 termination structure to form, preventing transcription of the operon

|
|
|
|
|
|
|
|
|
|
REGULATION OF GENE EXPRESSION |
91 |
||||||||||||||||||||
(a) Structure of the leader region |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A |
|
|
|
|
|
|
|
B |
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C |
D |
|
|
|
mRNA |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
trpE D C F B A |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
GAG/UAG repeats |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
(b) Protein synthesis with limiting tryptophan
C D
trpE D C F B A
A |
B |
(c) Protein synthesis with sufficient tryptophan
A
Termination
B
5
C D
Figure 3.22 Attenuation control of the trp operon in Bacillus subtilis. (a) The leader region contains two pairs of complementary sequences, enabling two alternative stem– loop structures A:B and C:D. The partial overlap of B and C prevents both structures forming. The leader also contains 11 repeats of GAG or UAG, which can bind TRAP (trp RNA-binding Attenuation Protein) in the presence of tryptophan. (b) In the absence of tryptophan, TRAP does not bind, the A:B stem–loop forms and the terminator C:D cannot form. Thus transcription of the operon occurs. (c) In the presence of tryptophan, TRAP binds to the GAG/UAG repeats, which blocks region A and prevents the formation of the A:B stem–loop. Region C is free to pair with region D to form the terminator structure, preventing transcription of the operon. Shaded circles indicate regions that are blocked from forming stem–loop structures
92 |
MOLECULAR GENETICS OF BACTERIA |
to these. Since the GAG/UAG repeats cover the whole of A, and part of B, the A:B stem–loop structure cannot form, which allows the formation of the C:D stem–loop terminator. Consequently transcription of the trp operon is prevented.
3. 2. 5 Two-component regulatory systems
The systems described above respond to conditions within the cell. Bacteria also have mechanisms for sensing and responding to external conditions and other stimuli, without such conditions altering the internal state of the cell. The mechanism for transmitting external signals to the interior of the cell is known as signal transduction.
One of the most common of such mechanisms are the two-component regulatory systems (Figure 3.23). In general, these systems comprise an integral membrane protein called a histidine protein kinase (HPK) and a separate cytoplasmic protein called a response regulator (RR). The HPK has two domains. The input domain is usually found on the outside of the cell in an ideal position to detect environmental signals. In contrast, the transmitter domain is located on the cytoplasmic face of the cell membrane, positioned to interact with the RR. When a stimulus causes a conformational change in HPK, the HPK autophosphorylates at a conserved histidine residue and subsequently transfers this phospho group to the response regulator. In this form the RR is able to bind to DNA to regulate transcription of the target genes.
The RR also consists of two domains: a receiver domain containing an aspartate residue which accepts the phospho group, and an output domain which can bind to DNA. Dephosphorylation of the response regulator is also important to terminate the signal and can be carried out by the RR itself or by a specific phosphatase. The above description outlines the orthodox scheme for this type of sensor. However, since the domains of the two component proteins are modular, the scheme is highly adaptable and a single response pathway can often contain multiple RRs and other variations on this simple scheme (Figure 3.23b). Although these are still often termed two-component systems because of the historical context, it is important to note that they will contain more than two components and are sometimes termed phosphorelay systems.
Over 300 two-component systems are now known in bacteria. E. coli and Ps. aeruginosa, for example, possess 32 and 89 RRs, and 30 and 55 HPKs, respectively. Indeed, there is so far only one known example of a bacterium (Mycoplasma genitalium) that does not contain these systems. Mycoplasmas are believed to have evolved into a minimal life form, containing just enough genes for replication and growth and have lost much regulatory capacity in order to reduce genome size.
REGULATION OF GENE EXPRESSION |
93 |
|||
(a) An orthodox two component system |
|
|
|
|
Input domain |
|
Stimulus |
|
|
|
|
|
|
|
Transmitter |
|
|
Histidine protein |
|
|
|
kinase |
|
|
domain |
|
|
|
|
|
|
|
|
|
Receiver |
|
P |
|
|
domain |
|
|
|
|
|
|
|
|
|
|
|
|
P |
|
Output |
|
|
|
|
domain |
Response |
P |
Gene |
|
|
regulator |
|
mRNA |
|
|
|
|
|
|
(b) Multi−step phosphorelay
One HPK initiates a phosphorelay that involves additional receiver domains
P
P
P
|
P |
P |
Gene |
|
mRNA |
Figure 3.23 Two component regulatory systems. (a) In an orthodox system, an external stimulus interacts with a specific membrane protein, causing a conformational change in the protein which activates autophosphorylation on the cytoplasmic face of the protein. The phosphate is then transferred to the receiver domain of a response regulator, enabling the output domain of the regulator to bind to operator sites of the regulated gene(s). (b) In a phosphorelay system, there is a chain of phosphorylation reactions, leading to the activated regulator protein
Amongst the wide variety of two-component systems responding to different stimuli, the HPK output domains are similar but the input domains, where the actual sensing mechanism resides, are very diverse. For example, the input domain from FixL (an HPK from Rhizobium) contains a haem group which responds to oxygen whilst the input domain from VirA from the plant pathogen Agrobacterium tumefaciens senses phenolic compounds released from plant
94 |
MOLECULAR GENETICS OF BACTERIA |
wounds. In Salmonella the pleiotropic PhoP-PhoQ system regulates virulence and acid tolerance but actually senses extracellular Mg2þ concentrations through the periplasmic input domain of the HPK, PhoQ, which contains several Mg2þ- binding acidic amino acids.
3. 2.6 Global regulatory systems
The regulatory mechanisms described may control not just a single operon but a very large number of unrelated genes. Such systems are referred to as global regulation. For example, the cAMP–CRP system, described above, is not just a regulator of the lac operon but affects the expression of some 200 genes. Other examples of global regulatory systems include the heat-shock response, acid response, oxidative stress response, cold shock response and osmotic stress response.
Many pathogenic bacteria also use global regulatory systems to control the expression of the mechanisms that they need to survive in the host and avoid the immune response. Bordetella species for example sense different stimuli including temperature, magnesium and nicotinic acid and globally regulate virulence gene expression in response to these stimuli.
The mechanisms involved in these global regulatory systems are very diverse and sometimes overlap to form networks which can cross-talk. The global heat shock response, for example is controlled by s32. This alternative s-factor regulates the expression of proteins known as molecular chaperones which can re-fold damaged proteins or degrade denatured proteins. Although these genes are often referred to as heat-shock genes, their expression can also be stimulated independently by a variety of other stress conditions, including bacteriophage infection. The heat shock response also overlaps substantially with the stringent response (see below) and with the SOS response referred to in Chapter 2 as the source of error-prone repair of DNA.
Another way in which cells can respond to changes in environmental conditions is by alteration in the degree of supercoiling of the DNA. In general, the overall level of supercoiling in the cell is controlled by a balance between the activities of DNA gyrase (which introduces negative supercoils) and DNA topoisomerase I (which removes supercoils). However, DNA topology is also influenced by the presence of histone-like proteins which bind to, and cause bending of, the DNA or even wrap it around them. Although the most abundant of these proteins, known as HU, is evenly distributed, others such as integration host factor (IHF), H-NS (histone-like nucleoid structuring protein) and FIS (Factor for Inversion Stimulation) are to some extent sequence specific and therefore affect the topology of DNA in defined regions.
If we combine this information with the knowledge that some promoters are affected by the degree of supercoiling of the DNA (some being stimulated
REGULATION OF GENE EXPRESSION |
95 |
by elevated supercoiling, while others prefer relaxed DNA), then it is clear that conditions, including anaerobiosis and changes in the osmolarity of the surrounding medium which affect either global or local supercoiling, may be expected to affect the expression of these genes.
3. 2.7 Feast or famine and the RpoS regulon
Until quite recently, most of the fundamental concepts of molecular genetics and metabolism were obtained using E. coli cells that were growing rapidly under laboratory conditions. However, in nature enteric bacteria like E. coli live under very variable nutritional conditions. As a consequence, evolution has optimized E. coli to be able to respond efficiently to conditions of both ‘feast and famine’. The transition from logarithmic growth to stationary phase, and starvation, is associated with metabolic and morphological changes that result in a remarkable resistance to a variety of stress conditions such as heat, acid, high salt and oxidative stress. The central regulator for many of these changes and for preparing the cell for famine and conditions of no growth is the alternative s-factor RpoS which controls the expression of 30 or more genes.
3. 2.8 Quorum sensing
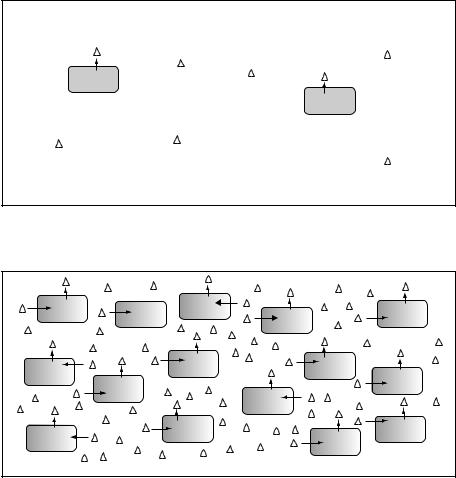
For many years bacteria have been considered merely as individual cells, yet as long ago as 1905 Erwin F. Smith wrote ‘The only explanation I can think of is that a multitude of bacteria are stronger than the few, and thus by union are able to overcome obstacles too great for the few’. This remarkably prophetic statement summarizes the phenomenon of quorum sensing, which only began to be unravelled in the 1980s, whereby bacteria measure and respond to their own population density. The principle, as illustrated by Figure 3.24, is that bacteria may both secrete and respond to a diffusible signal. At low cell density, the concentration of the signal in the surrounding medium is low, so the bacteria do not respond. When the cell density is high, the concentration of the signal is also high, and the bacteria respond by activating expression of a specific set of genes.
The phenomenon was originally uncovered following studies on the regulation of bioluminescence in marine Vibrio species. These species are able to live two separate life cycles, as free-living marine bacteria or as commensal bacteria occupying and providing bioluminescence in the light organs of fish and squid. Bioluminescence is an energetically expensive process and in these bacteria is tightly controlled by cell population density. So, when high numbers are present, as is the situation in the light organ, bioluminescence occurs, but when they are free living, isolated, cells it does not. In these species, the signal molecule, also called an autoinducer, is an acyl homoserine lactone which is synthesized by LuxI
96 |
MOLECULAR GENETICS OF BACTERIA |
(a) Low cell density Low level of signal Target genes off
(b) High cell density High level of signal Target genes on
Figure 3.24 Quorum sensing in bacteria. Some aspects of bacterial physiology can be influenced by the concentration of bacteria. This is mediated by sensing the concentration of a secreted signal (shown as triangles). (a) At a low cell density, the level of the signal in the environment will be low and the cells will not respond. (b) When the cell concentration is higher, the concentration of the signal will rise. This will be detected by the cells and the expression of the relevant genes will be activated
(acyl homoserine lactone synthase) and freely diffuses across the cell membrane into the environment surrounding the cell which has made it (Figure 3.25). If few cells are present, then production of this compound is limited and consequently it is not recognized as a signal. However, when many cells of the same species are present, the amount of acyl homoserine lactone in the environment (and hence
