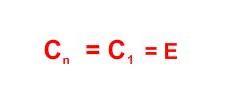- •Notation
- •[Edit]Schoenflies notation
- •[Edit]Hermann–Mauguin notation
- •[Edit]The correspondence between different notations
- •Igneous, metamorphic, sedimentary processes
- •Igneous Rock
- •Sedimentary Rock
- •Metamorphic Rock
- •The Rock Cycle
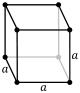
- •It is possible for an object to possess more
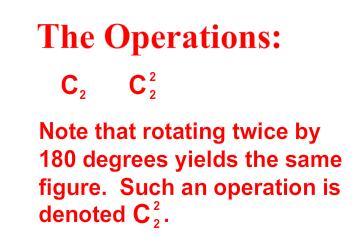
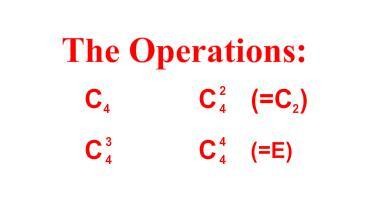
- •2 Major kinds of symmetry operations
Metamorphic Rock
Metamorphic rock, which comes from the Greek to "change form," is formed by applying great pressure and temperature to existing rock converting it into a new distinct type of rock. Igneous rocks, sedimentary rocks, and even other metamorphic rocks and be modified into metamorphic rocks.
Metamorphic rocks are usually created when they come under extreme pressure such as under many thousands of feet of bedrock or through being crushed at the junction of tectonic plates. Sedimentary rocks can become metamorphic rocks if the thousands of feet of sediments above them apply enough heat and pressure to further change the structure of the sedimentary rock.
Metamorphic rocks are harder than other types of rock so they're more resistant to weathering and erosion. Rock always converts into the same type of metamorphic rock. For example, the sedimentary rocks limestone and shale become marble and slate, respectively, when metamorphosed.
The Rock Cycle
We know that all three rock types can be turned into metamorphic rocks but all three types can also be changed through the rock cycle. All rocks can be weathered and eroded into sediments, which can then form sedimentary rock. Rocks can also be completely melted into magma and become reincarnated as igneous rock.
CRYSTAL STRUCTURE - Mutual arrangement of atoms, molecules or ions that are packed together in a crystal lattice to form a
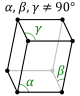
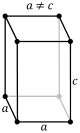
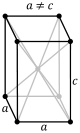
A crystal system is a class of point groups. Two point groups are placed in the same crystal system if the sets of possible lattice systems of their space groups are the same. For many point groups there is only one possible lattice system, and in these cases the crystal system corresponds to a lattice system and is given the same name. However, for the five point groups in the trigonal crystal class there are two possible lattice systems for their point groups: rhombohedral or hexagonal. In three dimensions there are seven crystal systems: triclinic, monoclinic, orthorhombic, tetragonal, trigonal, hexagonal, and cubic. The crystal system of a crystal or space group is determined by its point group but not always by its lattice.
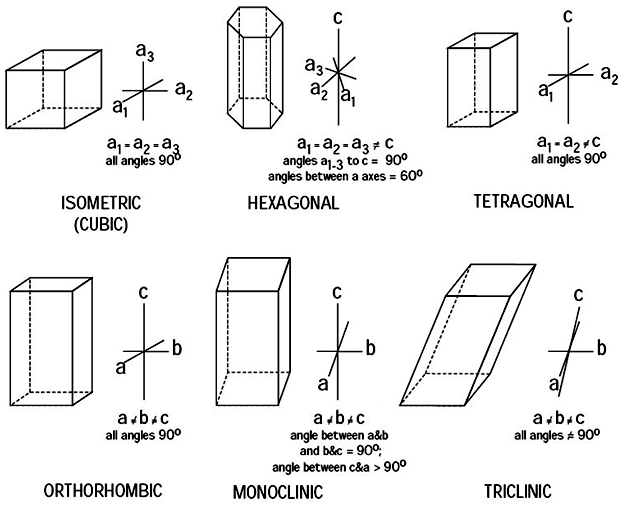
CRYSTAL SYSTEM - Atomic arrangement of the atoms of an element when it is in its solid state. In mineralogy, these are best classified in terms of their symmetry and correspond to the seven fundamental shapes for unit cells consistent with the 14 Bravais lattices. Six systems are recognized: isometric (cubic), hexagonal, tetragonal, orthorhombic, monoclinic, and triclinic.
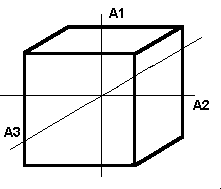
Minerals of the isometric system (also known as the cubic system) have mutually perpendicular crystallographic axes of equal length. All crystals have four 3-fold axes of symmetry diagonally from corner to corner through the center of the unit cell. They may also have up to three separate 4-fold axes of rotational symmetry from the center of each face through the origin to the center of the opposite face; these correspond to the crystallographic axes.
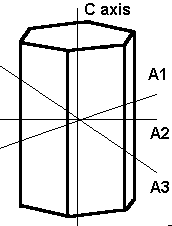
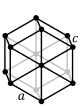
The hexagonal system have three crystallographic axes which intersect at 120° and a fourth which is perpendicular to the other three. This fourth axis is usually depicted vertically. The hexagonal system may be subdivided into hexagonal or trigonal divisions based upon whether the vertical axis has 6-fold or 3-fold rotational symmetry.
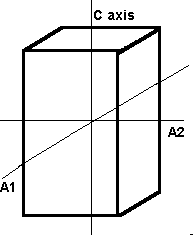
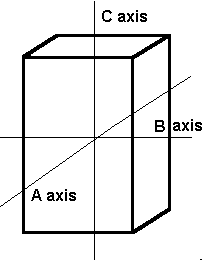
The tetragonal crystal system has three mutually perpendicular axes. The two horizontal axes are of equal length, while the vertical axis is of different length and may be either shorter or longer than the other two. Crystals all have a single 4-fold symmetry axis.
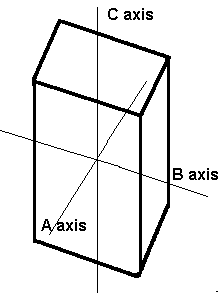
The orthorhombic system has three mutually perpendicular axes, each of which is of a different length than the others. Crystals of this system possess three 2-fold rotation axes and/or three mirror planes.
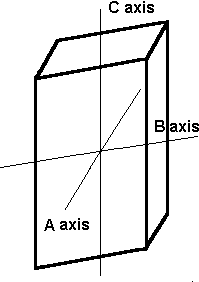
The monoclinic system has three unequal axes. Two axes are inclined toward each other at an oblique angle; these are usually depicted vertically. The third axis is perpendicular to the other two. The two vertical axes do not intersect one another at right angles, although both are perpendicular to the horizontal axis. Monoclinic crystals demonstrate a single 2-fold rotation axis and/or a single mirror plane.
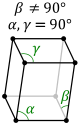
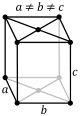
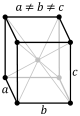
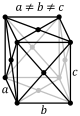
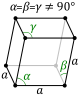
The triclinic system has three unequal axes, all of which intersect at oblique angles. None of the axes are perpendicular to any other axis. Crystals possess no symmetry at all.
|
Cubic The cube is composed of 6 square faces at 90 degree angles to each other. Each face intersects one of the crystallographic axes and is parallel to the other two. |
|
|
|
Tetragonal The tetragonal system also has three axes that all meet at 90°. It differs from the isometric system in that the C axis is longer than the A and B axis which are the same length. |
|
|
|
Hexagonal In the hexagonal system we have an additional axes, which gives the crystals six sides. Three of these are equal in length and meet at 60° to each other. The C or vertical axis is at 90° to the shorter axes. Mineralogists sometimes divide this into two systems, the hexagonal and the trigonal, based on their external appearance, as follows: |
|
|
|
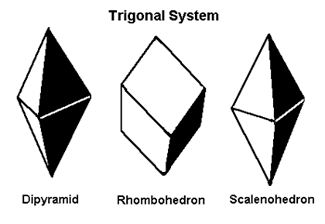
Trigonal Again, the trigonal system is a subsystem of the hexagonal. Most gem references will list these as hexagonal. |
|
|
|
Orthorhombic In this system there are three axes, all of which meet at 90° to each other. However, all the axes are a different length. |
|
|
|
Monoclinic . The above crystal systems all have axes sides that meet at 90°. In the monoclinic system all the axes are different lengths. Two of them, the A and C axes, meet at 90°, but the third one does not. |
|
|
|
Triclinic In this system all the axes are different lengths and none of them meet at 90°. |
|
|
|
The 7 lattice systems |
The 14 Bravais Lattices | |||
|
triclinic (parallelepiped) |
|
|
|
|
|
monoclinic (right prism with parallelogram base; here seen from above) |
simple |
base-centered |
|
|
|
|
|
|
| |
|
orthorhombic (cuboid) |
simple |
base-centered |
body-centered |
face-centered |
|
|
|
|
| |
|
tetragonal (square cuboid) |
simple |
body-centered |
|
|
|
|
|
|
| |
|
rhombohedral (trigonal trapezohedron) |
|
|
|
|
|
hexagonal (centered regular hexagon) |
|
|
|
|
|
cubic (isometric; cube) |
simple |
body-centered |
face-centered |
|
|
|
|
|
| |
|
Point Group Symmetry | |
|
Symmetry elements are points, lines or planes. Symmetry operations move the species (molecule or ion) about the symmetry element. Point Group Symmetry elements are those which coincide at the center (a point) of the species. The point group symmetry elements are: Mirrors Proper Axes of Rotation The Identity Inversion Center (Center of Symmetry) Improper Axes of Rotation
| |
|
As you read down this page, pay attention to the colors as well as the words, pictures and animations. The color blue will highlight discussion of symmetry elements. The color red will highlight discussion of symmetry operations.
| |
|
Mirrors | |
|
top |
Your right and left hands are identical by reflection through a mirror plane. Imagine σ as a plane pointing into the page. This mirror plane is the symmetry element. The motion of taking one hand through the plane to give its reflection is the symmetry operation.
|
|
top |
|
|
top |
|
|
Rotation Axes: Proper | |
|
top |
Consider a line perpendicular to this page through the oval. Rotating each hand about the axis by 180 degrees gives the other hand. Note that both are right hands. 360/180 = 2 = n So this is a two-fold axis of rotation. |
|
|
|
|
top |
A Three-fold axis of Rotation. The axis is perpendicular to the page through the triangle. 360/120 = 3 = n. |
|
top |
|
|
top |
A four-fold axis of rotation. 360/90 = 4 = n. Notice that every four-fold rotation axis contains a two-fold axis.
|
|
top |
A six-fold axis of rotation. 360/60 = 6 = n. Notice that every six-fold rotation axis contains a two-fold axis and a three-fold axis of rotation. |
|
top |
|
|
?-Fold axis of rotation. Proper axes of rotation of order 2, 3, 4, and 6 are most common. However, those of order 5, 7, and 8 are also observed. | |
|
The Identity | |
|
The special case of 360/360 = 1 = n is called the identity, E. If a molecule contains multiple axes of rotation, the axis with the highest value of n, the order, is called the principal axis. |
|
|
Inversion Center | |
|
top |
The center of symmetry, or inversion center, is a point through which the operation moves an atom at (x, y, z) to (-x, -y, -z). |
|
Rotation Axes: Improper | |
|
The improper axis of rotation is a rotation followed by a reflection. Shown here is a four-fold improper axis of rotation. The hands are rotated by 90 degrees, and then reflected through the mirror plane.
|
|
|
Every four-fold improper axis contains a two-fold proper axis of rotation. Always look down a proper axis of roation to see if an improper axis with order 2n is presesnt. |
|
|
top |
Shown here is a six-fold improper axis of rotation. The hands are rotated by 60 degrees, then reflected through the mirror plane. Every six-fold improper axis contains a three-fold proper axis of rotation. |
|
|
|
|
top |
Shown here is a three-fold improper axis of rotation. If a proper axis of rotation has aperpendicular mirror plane there will always be an improper axis of rotation of the same order.
|
Symmetry
A state in which parts on opposite sides of a plane, line, or point display arrangements that are related to one another via a symmetry operation such as translation, rotation, reflection or inversion.
Application of the symmetry operators leaves the entire crystal unchanged.
External Symmetry – definitions
Symmetry
Three main types of symmetry are recognized:
1 - Symmetry with respect to a point
2 - Symmetry with respect to a plane
3 - Symmetry with respect to a line