
Physical Properties of Aromatic Hydrocarbons
have a fragrant smell
generally less dense than water at 20°C
usually immiscible with water
soluble in organic solvents
Name |
Formula |
Boiling point (°C) |
Melting point (°C) |
Density at 20°C (g cm–3) |
Benzene |
|
80.1 |
5.5 |
0.878 |
Methylbenzene |
|
111 |
–95 |
0.867 |
Ethylbenzene |
|
136 |
–94 |
0.867 |
1,2-Dimethylbenzene |
|
144 |
–25.2 |
0.880 |
1,3-Dimethylbenzene |
|
139 |
–47.4 |
0.864 |
1,4-Dimethylbenzene |
|
138 |
13.3 |
0.861 |
5 Preparation of Benzene
Industrial Preparation
Catalytic Reforming of Alkanes
Catalytic reforming converts alkanes and cycloalkanes into aromatic hydrocarbons
e.g.
Pt
C 6H14
---®
C6H6
+ 4H2
6H14
---®
C6H6
+ 4H2
500°C, 10 – 20 atm
Destructive Distillation of Coal
Heating coal in the absence of air gives out coal gas, ammoniacal liquor, coal tar and coke
Coal tar is a mixture of many organic compounds, mainly aromatic ones
Benzene and methylbenzene can be obtained
Laboratory Synthesis
Decarboxylation of Sodium Salt of Benzoic Acid
When sodium benzoate is fused with sodium hydroxide, the carboxylate group is removed and benzene is formed
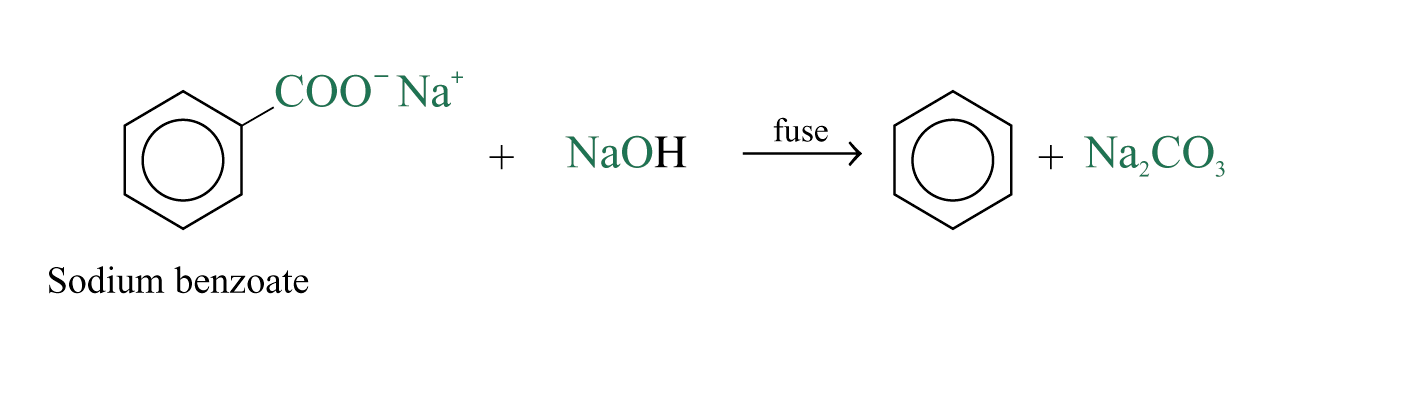
Reduction of Phenol
Phenol vapour is passed slowly over heated zinc dust to produce benzene and zinc(II) oxide

Trimerization of Alkyne
(lecture 5.3)
Preparation of Alkylbenzenes
Interaction of metallic Na with mixture of halogenalkanes and halogenbenzenes
![]()
6 Reactions of Benzene
Comparative Investigation of Chemical Properties of Cyclohexane, Cyclohexene and Benzene
Reaction |
Cyclohexane (a saturated alicyclic hydrocarbon) |
Cyclohexene (an unsaturated alicyclic hydrocarbon) |
Methylbenzene (an aromatic hydrocarbon) |
Action of Br2 in CH3Cl3 (in dark) |
No reaction |
Br2 decolourized and no HBr evolved |
No reaction with Br2 alone In the presence of FeBr3, Br2 decolourized and HBr fumes evolved |
Action of H2 (with Ni catalyst) |
No reaction |
1 mole of cyclohexene reacts with 1 mole of H2 at room temperature |
1 mole of methylbenzene reacts 3 moles of H2 at high temperature and pressure |
Action of acidified KMnO4 |
No reaction |
KMnO4 decolourized |
No reaction |
Action of conc. HNO3 and conc. H2SO4 |
No reaction |
Cyclohexene oxidized and colour darkens |
A yellow liquid is formed |
Methylbenzene is highly unsaturated, but it is resistant to oxidation and addition reactions
The resistance of oxidation and addition reactions of aromatic compounds is used to distinguish from unsaturated alkenes
Methylbenzene reacts with Br2 in the presence of FeBr3. It is through substitution reaction
Electrophilic Aromatic Substitution Reactions
Most characteristic reaction of aromatic compounds:
Electrophilic substitution reactions

The electrophiles attack the benzene ring, replacing one of the hydrogen atoms in the reaction
Electrophiles are either a positive ion (E+) or some other electron-deficient species with a partial positive charge (d+)
Mechanism of Electrophilic Aromatic Substitution Reactions
The electrophiles attack the benzene ring and unstable aromatic π- complex is formed.

A new C-E bond is formed in a slow, rate-limiting step to yield a nonaromatic carbocation intermediate, σ- complex:

The carbocation intermediate loses a proton and the neutral substitution prodact is formed.

Same Electrophilic Aromatic Substitututijn Reaction

Halogenation
Benzene reacts with chlorine and bromine in the presence of catalysts such as AlCl3, FeCl3, FeBr3, to give chlorobenzene and bromobenzene respectively


Nitration

Conc. H2SO4 increases the rate of reaction by increasing the concentration of the electrophile, NO2+ (nitronium ion)
Sulphonation
Benzene reacts with fuming sulphuric(VI) acid at room temperature to give benzenesulphonic acid
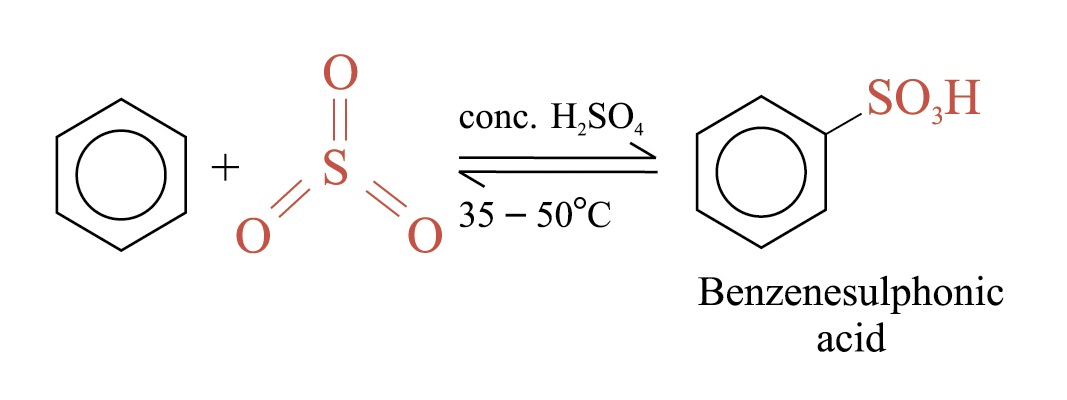
Heating aqueous solution of benzenesulphonic acid above 100°C, benzene and sulphuric(VI) acid are formed

Friedel–Crafts Alkylation
When benzene is warmed with a haloalkane in the presence of catalysts such as AlCl3, an alkylbenzene is formed

Aromatic ring must be at least as reactive as a halobenzene. Deactivated ring do not react.
Alkyl halide can be methyl, ethyl, 20, or 30; primary halides undergo carbocation rearrangemeht.
Friedel–Crafts Acylation
An acyl group, -COR is introduced onto the ring when an aromatic compound reacts with a carboxylic acid chloride or anhydrides

The mechanism of Friedel–Crafts Acylation is similar to that of Friedel–Crafts Alkylation
Substituent Effect in Substitututed Aromatic Ring
Substitutents already present on the ring have tow effects:
Substitutents effect the reactivity of the aromatic ring. Some substitutents activate the ring, making it more reactive than benzene, and some deactivate the ring making it less reactive than benzene.
Substitutents effect the orientation of the reaction. The nature of the substitutent already present on the benzene ring determines the position of the second substitutent.
Classification of Substitutuent Effect in Electrophilic Aroma Aromatic Substitutution
Substituent can be classified into three groups:
Reactivity


Trisubstituted Benzenes: Additivity of Effect
If the directing effects of tow groups reinforce each other:

If the directing effects of tow groups oppose each other, the more powerful activation group has the dominant influence, but mixtures of products often esult.

If there are two different deactivatorу substituents, more powerful deactivator directs next substituent to meta- position

Further rarely occurs between two groups in a meta- disubstituted compound because this site is too hindered.

Nucleophilic aromatic substitution
Aryls that have strong electron-withdrawing substituents can undergo nucleophilic aromatic substitution

Aryl halides that have electron-withdrawing substituents in a position ortho- or para- to the halogen can undergo nucleophilic aromatic substitution

Aryl halides without electron-withdrawing substituents are inert to nucleophiles under most condition.
At high temperature and pressure chlorobenzene can be forced to react.

Oxidation of alkylbenzene side chains
Aromatic rings are inert to oxidation under most condition. Alkylbenzenes are oxidized to benzoic acid by strong oxidizing agents such as hot alkaline potassium manganate(VII)

Examples:



The C = C double bond and acyl groups in the side chain are oxidized by hot alkaline potassium manganate(VII)
e.g.

Ozonolysis
Like alkenes, aromatic compounds can undergo oxidation reaction by ozone.
Ozonolysis is a widely used method for locating the subtituents of benzene

Redaction of aromatic compounds
Aromatic rings are inert to catalytic hydrogenation under conditions that reduce typical alkene double bond. As a result, it’s possible to selectively reduce alkene double bonds in the presence of aromatic ring:

To hydrogenate an aromatic ring, it’s necessary either to use a platinum catalyst with hydrogen gas at several hundred atmospheres pressure or to use a more powerful catalyst such as rhodium on carbon.





