
The effect of surface area on reaction rates
This page describes and explains the effect of changing the surface area of a solid on the rate of a reaction it is involved in. This applies to reactions involving a solid and a gas, or a solid and a liquid. It includes cases where the solid is acting as a catalyst.
The facts
What happens?
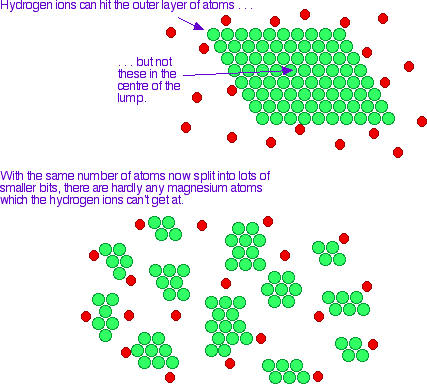
The more finely divided the solid is, the faster the reaction happens. A powdered solid will normally produce a faster reaction than if the same mass is present as a single lump. The powdered solid has a greater surface area than the single lump.
Note: Why normally? What exceptions can there be?
Imagine a case of a very fine powder reacting with a gas. If the powder was in one big heap, the gas may not be able to penetrate it. That means that its effective surface area is much the same as (or even less than) it would be if it were present in a single lump.
A small heap of fine magnesium powder tends to burn rather more slowly than a strip of magnesium ribbon, for example.
Some examples
Calcium carbonate and hydrochloric acid
In the lab, powdered calcium carbonate reacts much faster with dilute hydrochloric acid than if the same mass was present as lumps of marble or limestone.
![]()
The catalytic decomposition of hydrogen peroxide
This is another familiar lab reaction. Solid manganese(IV) oxide is often used as the catalyst. Oxygen is given off much faster if the catalyst is present as a powder than as the same mass of granules.
![]()
Catalytic converters
Catalytic converters use metals like platinum, palladium and rhodium to convert poisonous compounds in vehicle exhausts into less harmful things. For example, a reaction which removes both carbon monoxide and an oxide of nitrogen is:
![]()
Because the exhaust gases are only in contact with the catalyst for a very short time, the reactions have to be very fast. The extremely expensive metals used as the catalyst are coated as a very thin layer onto a ceramic honeycomb structure to maximise the surface area.
The explanation
You are only going to get a reaction if the particles in the gas or liquid collide with the particles in the solid. Increasing the surface area of the solid increases the chances of collision taking place.
Imagine a reaction between magnesium metal and a dilute acid like hydrochloric acid. The reaction involves collision between magnesium atoms and hydrogen ions.
Increasing the number of collisions per second increases the rate of reaction.
![]()
The effect of concentration on reaction rates
This page describes and explains the way that changing the concentration of a solution affects the rate of a reaction. Be aware that this is an introductory page only. If you are interested in orders of reaction, you will find separate pages dealing with these. You can access these via the rates of reaction menu (link at the bottom of the page).
The facts
What happens?
For many reactions involving liquids or gases, increasing the concentration of the reactants increases the rate of reaction. In a few cases, increasing the concentration of one of the reactants may have little noticeable effect of the rate. These cases are discussed and explained further down this page.
Don't assume that if you double the concentration of one of the reactants that you will double the rate of the reaction. It may happen like that, but the relationship may well be more complicated.
Note: The mathematical relationship between concentration and rate of reaction is dealt with on the page about orders of reaction. If you are interested, you can use this link or read about it later via the rate of reaction menu (link at the bottom of the page).
Some examples
The examples on this page all involve solutions. Changing the concentration of a gas is achieved by changing its pressure. This is covered on a separate page.
Note: If you want to explore the effect of changing pressure on the rate of a reaction, you could use this link. Alternatively, use the link to the rates of reaction menu at the bottom of this page.
Zinc and hydrochloric acid
In the lab, zinc granules react fairly slowly with dilute hydrochloric acid, but much faster if the acid is concentrated.
![]()
The catalytic decomposition of hydrogen peroxide
Solid manganese(IV) oxide is often used as a catalyst in this reaction. Oxygen is given off much faster if the hydrogen peroxide is concentrated than if it is dilute.
![]()
The reaction between sodium thiosulphate solution and hydrochloric acid
This is a reaction which is often used to explore the relationship between concentration and rate of reaction in introductory courses (like GCSE). When a dilute acid is added to sodium thiosulphate solution, a pale yellow precipitate of sulphur is formed.
![]()
As the sodium thiosulphate solution is diluted more and more, the precipitate takes longer and longer to form.
The explanation
Cases where changing the concentration affects the rate of the reaction
This is the common case, and is easily explained.
Collisions involving two particles
The same argument applies whether the reaction involves collision between two different particles or two of the same particle.
In order for any reaction to happen, those particles must first collide. This is true whether both particles are in solution, or whether one is in solution and the other a solid. If the concentration is higher, the chances of collision are greater.
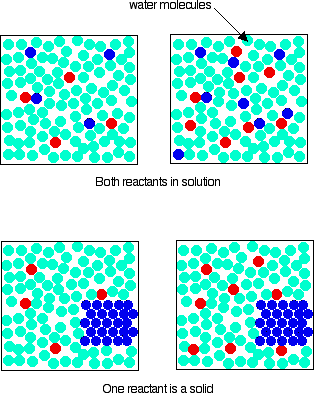
Reactions involving only one particle
If a reaction only involves a single particle splitting up in some way, then the number of collisions is irrelevant. What matters now is how many of the particles have enough energy to react at any one time.
Note: If you aren't sure about this, then read the page about collision theory and activation energy before you go on. Use the BACK button on your browser to return to this page.
Suppose that at any one time 1 in a million particles have enough energy to equal or exceed the activation energy. If you had 100 million particles, 100 of them would react. If you had 200 million particles in the same volume, 200 of them would now react. The rate of reaction has doubled by doubling the concentration.
Cases where changing the concentration doesn't affect the rate of the reaction
At first glance this seems very surprising!
Where a catalyst is already working as fast as it can
Suppose you are using a small amount of a solid catalyst in a reaction, and a high enough concentration of reactant in solution so that the catalyst surface was totally cluttered up with reacting particles.
Increasing the concentration of the solution even more can't have any effect because the catalyst is already working at its maximum capacity.
