
- •1.The main characteristics of atomic nucleus
- •10. Macroscopic cross section
- •19.Nuclear reactions by alpha particles.
- •28.Total cross sections
- •5) Features of fission reactions with charged particles
- •32) Division scheme of fuel in the reactors with slow neutrons
- •25. The differential cross section
- •34.Coulomb barrier
- •37.The laws of conservation angular momentum, parity, charge, baryon charge, isospin.
- •8. The cross section for Photodisintegration Reaction.
- •26. Integrated cross section
- •35. Direct Nuclear Reaction
- •21.Nuclear reaction induced by heavy protons.
- •30.The Rutherford cross section
- •3. The main types of interactions in the microphysics
- •12. Collisions of neutrons in the reactor core
- •15. Nuclear reaction induced by neutrons
- •Control
2. Parameters of nuclear systems in radioactive decay. Radioactive decay, also known as nuclear decay or radioactivity, is the process by which a nucleus of an unstable atom loses energy by emitting particles of ionizing radiation. A material that spontaneously emits this kind of radiation—which includes the emission of energetic alpha particles, beta particles, and gamma rays—is considered radioactive. As for types of radioactive radiation, it was found that an electric or magnetic field could split such emissions into three types of beams. The rays were given the alphabetic names alpha, beta, and gamma, in order of their ability to penetrate matter. While alpha decay was seen only in heavier elements (atomic number 52, tellurium, and greater), the other two types of decay were seen in all of the elements. Lead (atomic number 82) is the heaviest element to have any isotopes stable (to the limit of measurement) to radioactive decay. Radioactive decay is seen in all isotopes of all elements of atomic number 83 (bismuth) or greater (bismuth, however, is only very slightly radioactive). The decay rate, or activity, of a radioactive substance is characterized by:
Constant quantities: 1)The half-life—t1/2, is the time taken for the activity of a given amount of a radioactive substance to decay to half of its initial value; 2)The decay constant— λ, "lambda" the inverse of the mean lifetime. 3)The mean lifetime— τ, "tau" the average lifetime of a radioactive particle before decay.
11. Physics of neutron diffusion. Neutron transport is the study of the motions and interactions of neutrons with materials. Nuclear scientists and engineers often need to know where neutrons are in an apparatus, what direction they are going, and how quickly they are moving. It is commonly used to determine the behavior of nuclear reactor cores and experimental or industrial neutron beams. Neutron transport is a type of radiative transport. The neutron transport equation is a balance statement that conserves neutrons. Each term represents a gain or a loss of a neutron, and the balance, in essence, claims that neutrons gained equals neutrons lost. It is formulated as follows

20. Nuclear reaction induced by neutrinos. A neutrino is an electrically neutral, weakly interacting elementary subatomic particle with half-integer spin. The neutrino (meaning "small neutral one" in Italian) is denoted by the Greek letter ν (nu). All evidence suggests that neutrinos have mass but that their mass is tiny even by the standards of subatomic particles. Their mass has never been measured accurately. Neutrinos do not carry electric charge, which means that they are not affected by the electromagnetic forces that act on charged particles such as electrons and protons. Neutrinos are affected only by the weak sub-atomic force, of much shorter range than electromagnetism, and gravity, which is relatively weak on the subatomic scale. Therefore a typical neutrino passes through normal matter unimpeded. Neutrinos can interact with a nucleus, changing it to another nucleus. This process is used in radiochemical neutrino detectors. In this case, the energy levels and spin states within the target nucleus have to be taken into account to estimate the probability for an interaction. In general the interaction probability increases with the number of neutrons and protons within a nucleus. Very much like neutrons do in nuclear reactors, neutrinos can induce fission reactions within heavy nuclei. So far, this reaction has not been measured in a laboratory, but is predicted to happen within stars and supernovae. The process affects the abundance of isotopes seen in the universe. Neutrino fission of deuterium nuclei has been observed in the Sudbury Neutrino Observatory, which uses a heavy water detector.
29. The threshold of the nuclear reaction. In particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles must have when they collide. The threshold energy is always greater than or equal to the rest energy of the desired particle. In most cases, since momentum is also conserved, the threshold energy is significantly greater than the rest energy of the desired particle - and thus there will still be considerable kinetic energy in the final particles. The threshold displacement energy Td is the minimum kinetic energy that an atom in a solid needs to be permanently displaced from its lattice site to a defect position. It is also known as "displacement threshold energy" or just "displacement energy". In a crystal, a separate threshold displacement energy exists for each crystallographic direction. Then one should distinguish between the minimum Td(min) and average Td(ave) over all lattice directions threshold displacement energies. In amorphous solids it may be possible to define an effective displacement energy to describe some other average quantity of interest. Threshold displacement energies in typical solids are of the order of 10 - 50 eV.
40. The ratio between the elastic and inelastic cross. Elastic scattering is a form of particle scattering in scattering theory, nuclear physics and particle physics. In this process, the kinetic energy of a particle is conserved in the center-of-mass frame, but their direction of propagation is modified (by interaction with other particles and/or potentials). During elastic scattering of high-energy subatomic particles, linear energy transfer (LET) takes place until the incident particle's energy and speed has been reduced to the same as its surroundings, at which point the particle is "stopped."
In chemistry, nuclear physics, and particle physics, inelastic scattering is a fundamental scattering process in which the kinetic energy of an incident particle is not conserved (in contrast to elastic scattering). In an inelastic scattering process, some of the energy of the incident particle is lost or increased. Although the term is historically related to the concept of inelastic collision in dynamics, the two concepts are quite distinct; the latter refers to processes in which the total kinetic energy is not conserved. In general, scattering due to inelastic collisions will be inelastic, but, since elastic collisions often transfer kinetic energy between particles, scattering due to elastic collisions can also be inelastic, as in Compton scattering.
1.The main characteristics of atomic nucleus
The central region of an atom. Atoms are composed of negatively charged electrons, positively charged protons, and electrically neutral neutrons. The protons and neutrons (collectively known as nucleons) are located in a small central region known as the nucleus. The electrons move in orbits which are large in comparison with the dimensions of the nucleus itself. Protons and neutrons possess approximately equal masses, each roughly 1840 times that of an electron. The number of nucleons in a nucleus is given by the mass number A and the number of protons by the atomic number Z. Nuclear radii r are given approximately by r = 1.2 × 10-15 m A1/3.
For example the hydrogen nucleus includes one proton, the oxygen nucleus - eight, and the silver nucleus - forty-seven. The number of the electrons rotating around the nucleus is the same as the number of the protons in that nucleus. And so around the hydrogen nucleus there is one electron rotating, around the oxygen nucleus - eight, and around the silver nucleus - forty-seven. The mass of the nucleus consist of the masses of all the protons and neutrons summed together. In the periodic table the mass of the nucleus is represented with the mass number A. So if a nucleus consists of 5 protons and 5 neutrons then the atomic number is equal to 5, and the mass number is equal to 10. And vice versa an atom of Z=7 and A=16 consists of 7 protons, 9 neutrons, and 7 electrons. As it was mentioned the nuclei of various elements consist of various numbers of protons. And what about the neutrons? Well, the nuclei of one element (of one Z number) can consist of various numbers of neutrons!
10. Macroscopic cross section
The nuclear cross section of a nucleus is used to characterize the probabilitythat a nuclear reaction will occur. The concept of a nuclear cross section can be quantified physically in terms of "characteristic area" where a larger area means a larger probability of interaction. The standard unit for measuring a nuclear cross section (denoted as σ) is the barn, which is equal to 10−28 m² or 10−24 cm². Cross sections can be measured for all possible interaction processes together, in which case they are called total cross sections, or for specific processes, distinguishing elastic scatteringandinelastic scattering; of the latter, amongstneutron cross sectionstheabsorption cross sections are of particular interest.
Nuclear cross sections are used in determining the nuclear reactionrate, and are governed by the reaction rate equation for a particular set of particles (usually viewed as a "beam and target" thought experiment where one particle or nucleus is the "target" [typically at rest] and the other is treated as a "beam" [projectile with a given energy]).
For neutron interactions incident upon a thin sheet of material (ideally made of a single type of isotope), the nuclear reaction rate equation is written as:
![]()
where:
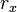 :
number of reactions of type x, units: [1/time/volume]
:
number of reactions of type x, units: [1/time/volume] :
neutron beam flux, units: [1/area/time]
:
neutron beam flux, units: [1/area/time]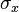 :
microscopic cross section for reaction
:
microscopic cross section for reaction 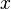 ,
units: [area] (usuallybarns or
cm2).
,
units: [area] (usuallybarns or
cm2).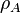 :
density of atoms in the target in units of [1/volume]
:
density of atoms in the target in units of [1/volume]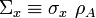 :
macroscopic cross-section [1/length]
:
macroscopic cross-section [1/length]
Types
of reactions frequently encountered are s:
scattering, ![]() :
radiative capture,a:
absorption (radiative capture belongs to this type), f:
fission, the corresponding notation for cross-sections
being:
:
radiative capture,a:
absorption (radiative capture belongs to this type), f:
fission, the corresponding notation for cross-sections
being: ![]() ,
,![]() ,
,![]() ,
etc. A special case is the total cross-section
,
etc. A special case is the total cross-section![]() ,
which gives the probability of a neutron to undergo any sort of
reaction (
,
which gives the probability of a neutron to undergo any sort of
reaction (![]() ).
).
Formally, the equation above defines the macroscopic neutron cross-section (for reaction x) as the proportionality constant between a neutron flux incident on a (thin) piece of material and the number of reactions that occur (per unit volume) in that material. The distinction between macroscopic and microscopic cross-section is that the former is a property of a specific lump of material (with its density), while the latter is an intrinsic property of a type of nuclei.
