
- •Preface to the 3rd edition
- •General Pharmacology
- •Systems Pharmacology
- •Therapy of Selected Diseases
- •Subject Index
- •Abbreviations
- •General Pharmacology
- •History of Pharmacology
- •Drug and Active Principle
- •The Aims of Isolating Active Principles
- •European Plants as Sources of Effective Medicines
- •Drug Development
- •Congeneric Drugs and Name Diversity
- •Oral Dosage Forms
- •Drug Administration by Inhalation
- •Dermatological Agents
- •From Application to Distribution in the Body
- •Potential Targets of Drug Action
- •External Barriers of the Body
- •Blood–Tissue Barriers
- •Membrane Permeation
- •Binding to Plasma Proteins
- •The Liver as an Excretory Organ
- •Biotransformation of Drugs
- •Drug Metabolism by Cytochrome P450
- •The Kidney as an Excretory Organ
- •Presystemic Elimination
- •Drug Concentration in the Body as a Function of Time—First Order (Exponential) Rate Processes
- •Time Course of Drug Concentration in Plasma
- •Time Course of Drug Plasma Levels during Repeated Dosing (A)
- •Time Course of Drug Plasma Levels during Irregular Intake (B)
- •Accumulation: Dose, Dose Interval, and Plasma Level Fluctuation (A)
- •Dose–Response Relationship
- •Concentration–Effect Curves (B)
- •Concentration–Binding Curves
- •Types of Binding Forces
- •Agonists—Antagonists
- •Other Forms of Antagonism
- •Enantioselectivity of Drug Action
- •Receptor Types
- •Undesirable Drug Effects, Side Effects
- •Drug Allergy
- •Cutaneous Reactions
- •Drug Toxicity in Pregnancy and Lactation
- •Pharmacogenetics
- •Placebo (A)
- •Systems Pharmacology
- •Sympathetic Nervous System
- •Structure of the Sympathetic Nervous System
- •Adrenergic Synapse
- •Adrenoceptor Subtypes and Catecholamine Actions
- •Smooth Muscle Effects
- •Cardiostimulation
- •Metabolic Effects
- •Structure–Activity Relationships of Sympathomimetics
- •Indirect Sympathomimetics
- •Types of
- •Antiadrenergics
- •Parasympathetic Nervous System
- •Cholinergic Synapse
- •Parasympathomimetics
- •Parasympatholytics
- •Actions of Nicotine
- •Localization of Nicotinic ACh Receptors
- •Effects of Nicotine on Body Function
- •Aids for Smoking Cessation
- •Consequences of Tobacco Smoking
- •Dopamine
- •Histamine Effects and Their Pharmacological Properties
- •Serotonin
- •Vasodilators—Overview
- •Organic Nitrates
- •Calcium Antagonists
- •ACE Inhibitors
- •Drugs Used to Influence Smooth Muscle Organs
- •Cardiac Drugs
- •Cardiac Glycosides
- •Antiarrhythmic Drugs
- •Drugs for the Treatment of Anemias
- •Iron Compounds
- •Prophylaxis and Therapy of Thromboses
- •Possibilities for Interference (B)
- •Heparin (A)
- •Hirudin and Derivatives (B)
- •Fibrinolytics
- •Intra-arterial Thrombus Formation (A)
- •Formation, Activation, and Aggregation of Platelets (B)
- •Inhibitors of Platelet Aggregation (A)
- •Presystemic Effect of ASA
- •Plasma Volume Expanders
- •Lipid-lowering Agents
- •Diuretics—An Overview
- •NaCl Reabsorption in the Kidney (A)
- •Aquaporins (AQP)
- •Osmotic Diuretics (B)
- •Diuretics of the Sulfonamide Type
- •Potassium-sparing Diuretics (A)
- •Vasopressin and Derivatives (B)
- •Drugs for Gastric and Duodenal Ulcers
- •Laxatives
- •Antidiarrheal Agents
- •Drugs Affecting Motor Function
- •Muscle Relaxants
- •Nondepolarizing Muscle Relaxants
- •Depolarizing Muscle Relaxants
- •Antiparkinsonian Drugs
- •Antiepileptics
- •Pain Mechanisms and Pathways
- •Eicosanoids
- •Antipyretic Analgesics
- •Nonsteroidal Anti-inflammatory Drugs (NSAIDs)
- •Cyclooxygenase (COX) Inhibitors
- •Local Anesthetics
- •Opioid Analgesics—Morphine Type
- •General Anesthesia and General Anesthetic Drugs
- •Inhalational Anesthetics
- •Injectable Anesthetics
- •Sedatives, Hypnotics
- •Benzodiazepines
- •Pharmacokinetics of Benzodiazepines
- •Therapy of Depressive Illness
- •Mania
- •Therapy of Schizophrenia
- •Psychotomimetics (Psychedelics, Hallucinogens)
- •Hypothalamic and Hypophyseal Hormones
- •Thyroid Hormone Therapy
- •Glucocorticoid Therapy
- •Follicular Growth and Ovulation, Estrogen and Progestin Production
- •Oral Contraceptives
- •Antiestrogen and Antiprogestin Active Principles
- •Aromatase Inhibitors
- •Insulin Formulations
- •Treatment of Insulin-dependent Diabetes Mellitus
- •Treatment of Maturity-Onset (Type II) Diabetes Mellitus
- •Oral Antidiabetics
- •Drugs for Maintaining Calcium Homeostasis
- •Drugs for Treating Bacterial Infections
- •Inhibitors of Cell Wall Synthesis
- •Inhibitors of Tetrahydrofolate Synthesis
- •Inhibitors of DNA Function
- •Inhibitors of Protein Synthesis
- •Drugs for Treating Mycobacterial Infections
- •Drugs Used in the Treatment of Fungal Infections
- •Chemotherapy of Viral Infections
- •Drugs for the Treatment of AIDS
- •Drugs for Treating Endoparasitic and Ectoparasitic Infestations
- •Antimalarials
- •Other Tropical Diseases
- •Chemotherapy of Malignant Tumors
- •Targeting of Antineoplastic Drug Action (A)
- •Mechanisms of Resistance to Cytostatics (B)
- •Inhibition of Immune Responses
- •Antidotes and Treatment of Poisonings
- •Therapy of Selected Diseases
- •Hypertension
- •Angina Pectoris
- •Antianginal Drugs
- •Acute Coronary Syndrome— Myocardial Infarction
- •Congestive Heart Failure
- •Hypotension
- •Gout
- •Obesity—Sequelae and Therapeutic Approaches
- •Osteoporosis
- •Rheumatoid Arthritis
- •Migraine
- •Common Cold
- •Atopy and Antiallergic Therapy
- •Bronchial Asthma
- •Emesis
- •Alcohol Abuse
- •Local Treatment of Glaucoma
- •Further Reading
- •Further Reading
- •Picture Credits
- •Drug Indexes

140 Antianemics
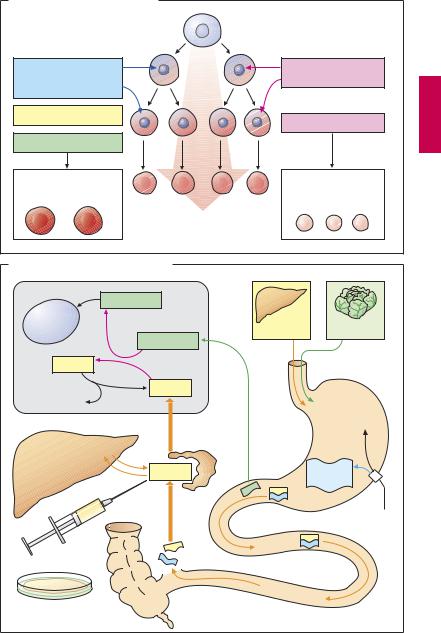
Drugs for the Treatment of Anemias
Anemia denotes a reduction in red blood cell count or hemoglobin content, or both.
Erythropoiesis (A)
Blood corpuscles develop from stem cells through several cell divisions (n = 17!). Hemoglobin is then synthesized and the cell nucleus is extruded. Erythropoiesis is stimulated by the hormone erythropoietin (a glycoprotein), which is released from the kidneys when renal oxygen tension declines. A nephrogenic anemia can be ameliorated by parenteral administration of recombinant erythropoietin (epoetin alfa) or hyperglycosylated erythropoietin (darbepoetin; longer half-life than epoetin).
Even in healthy humans, formation of red blood cells and, hence, the oxygen transport capacity of blood, is augmented by erythropoietin,. This effect is equivalent to high-al- titude training and is employed as a doping method by high-performance athletes. Erythropoietin is inactivated by cleavage of sugar residues, with a biological half-life of ~ 5 hours after intravenous injection and a t½
>20 hours after subcutaneous injection. Given adequate production of erythro-
poietin, a disturbance of erythropoiesis is due to two principal causes. (1) Cell multiplication is inhibited because DNA synthesis is insuf cient. This occurs in deficiencies of vitamin B12 or folic acid(macrocytichyperchromic anemia). (2) Hemoglobin synthesis is impaired. This situation arises in iron deficiency, since Fe2+ is a constituent of hemoglobin (microcytic hypochromic anemia).
Vitamin B12 (B)
Vitamin B12 (cyanocobalamin) is produced by bacteria; vitamin B12 generated in the colon, however, is unavailable for absorption. Liver, meat, fish, and milk products are rich sources of the vitamin. The minimal requirement is about 1 µg/day. Enteral absorption ofvitamin B12 requirestheso-called “intrinsic factor” from parietal cells of the stomach. The complex formed with this gly-
coprotein undergoes endocytosis in the ileum. Bound to its transport protein, transcobalamin, vitamin B12 is destined for storage in the liver or uptake into tissues.
A frequent cause of vitamin B12 deficiency is atrophic gastritis leading to a lack of intrinsic factor. Besides megaloblastic anemia, damage to mucosal linings and degeneration of myelin sheaths with neurological sequelae will occur (pernicious anemia). The optimal therapy consists in parenteral administration of cyanocobalamin or hydroxycobalamin (vitamin B12a; exchange of –CN for –OH group). Adverse effects, in the form of hypersensitivity reactions, are very rare.
Folic Acid (B)
Leafy vegetables and liver are rich in folic acid (FA). The minimal requirement is
~ 50 µg/day. Polyglutamine-FA in food is hydrolyzed to monoglutamine-FA prior to being absorbed. Causes of deficiency include insuf cient intake, malabsorption, and increased requirements during pregnancy (hence the prophylactic administration during pregnancy). Antiepileptic drugs and oral contraceptives may decrease FA absorption, presumably by inhibiting the formation of monoglutamine-FA. Inhibition of dihydro-FAreductase (e.g., by methotrexate, p.300) depresses the formation of the active species, tetrahydro-FA. Symptoms of deficiency are megaloblastic anemia and mucosal damage. Therapy consists in oral administration of FA.
Administration of FA can mask a vitamin B12 deficiency. Vitamin B12 isrequired for the conversion of methyltetrahydro-FA to tetra- hydro-FA, which is important for DNA-syn- thesis (B). Inhibition of this reaction due to vitamin B12 deficiency can be compensated by increased FA intake. The anemia is readily corrected; however, nerve degeneration progresses unchecked and its cause is made more dif cult to diagnose by the absence of hematologicalchanges. Indiscriminate use of FA-containing multivitamin preparations can, therefore, be harmful.
Drugs for the Treatment of Anemias |
141 |
A. Erythropoiesis in bone marrow |
|
|
|
Inhibition of DNA |
Erythropoetin |
Inhibition of |
|
synthesis |
hemoglobin synthesis |
||
(cell multiplication) |
|
||
Vit. B12 deficiency |
Iron deficiency |
||
|
|||
|
|
||
Folate deficiency |
|
|
|
Very few large |
|
Few small |
|
hemoglobin-rich |
|
hemoglobin-poor |
|
erythrocytes |
|
erythrocytes |
B. Vitamin B12 and folate metabolism |
|
|
|
Folic acid H4 |
|
|
|
DNA |
|
|
|
synthesis |
|
Vit. B12 |
Folic acid |
H3C- |
Folic acid H4 |
|
|
H3C- Vit. B12 |
|
|
|
|
Vit. B12 |
|
|
H3C- |
|
|
|
|
Trans- |
|
HCl |
|
|
|
|
|
cobalamin II |
|
|
Storage supply for |
|
|
|
3 years |
Vit. B12 |
|
Intrinsic |
|
|
||
|
|
|
factor |
|
|
|
Parietal cell |
i.m. |
|
|
|
Streptomyces |
|
|
|
griseus |
|
|
|

142 Antianemics
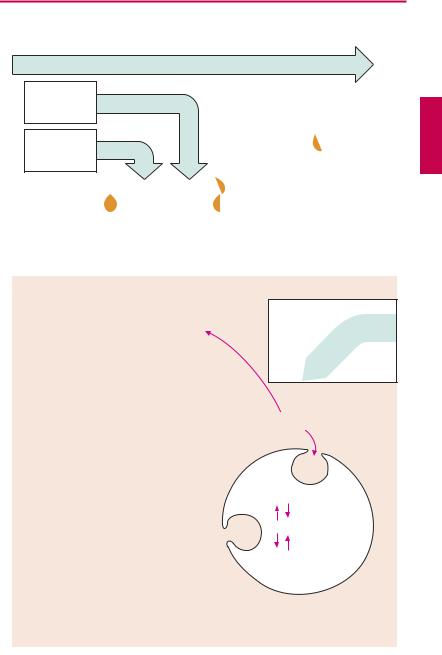
Iron Compounds
Not all iron ingested in food is equally absorbable. Trivalent Fe3+ is virtually not taken up from the neutral milieu of the small bowel, where the divalent Fe2+ is markedly better absorbed. Uptake is particularly ef cient in the form of heme (present in hemoglobin and myoglobin). Within the mucosal cells of the gut, iron is oxidized and either deposited as ferritin (see below) or passed on to the transport protein, transferrin. The amount absorbed does not exceed that needed to balance losses due to epithelial shedding from skin and mucosae or hemorrhage (socalled“mucosal block”). Inmenthisamount is ~ 1 mg/day, in women it is ~ 2 mg/day (because of menstrual blood loss); it corresponds to about 10% of the dietary intake. The transferrin–iron complex undergoes endocytotic uptake into erythrocyte precursors to be utilized for hemoglobin synthesis. About 70% of the total body store of iron (~ 5 g) is contained within erythrocytes. When these are degraded by macrophages of the mononuclear phagocyte system, iron is liberated from hemoglobin. Fe3+can be stored as ferritin (= protein apoferritin + Fe3+) or be returned to erythropoiesis sites via transferrin.
A frequent cause of iron deficiency is chronic blood loss due to gastric/intestinal ulcers or tumors. One liter of blood contains 500 mg of iron in healthy condition. Despite a significant increase in absorption rate, absorption isunable to keep up with lossesand the body store of iron falls. Iron deficiency results in impaired synthesis of hemoglobin and anemia.
The treatment of choice (after the cause of bleeding has been found and eliminated) consists in the oral administration of Fe2+- compounds, e.g., ferrous sulfate (daily dose 100 mg of iron, equivalent to 300 mg of FeSO4, divided into multiple doses). Replenishing of iron stores may take several months. Oral administration, however, is ad-
vantageous in that it is impossible to overload the body with iron through an intact mucosa because of its demand-regulated absorption (mucosal block).
Adverse effects. The frequent gastrointestinal complaints (epigastric pain, diarrhea, constipation) necessitate intake ofiron preparations with or after meals, although absorption is higher from the empty stomach.
Interactions. Antacids inhibit iron absorption. Combination with ascorbic acid (vitamin C) to protect Fe2+ from oxidation to Fe3+ is theoretically sound but practically is not needed.
Parenteral administration of Fe3+ salts is indicated only when adequate oral replacement is not possible. There is a risk of overdosage, with iron deposition in tissues (hemosiderosis). The binding capacity of transferrin is limited and free Fe3+ is toxic. Therefore, Fe3+complexes are employed that can donate Fe3+ directly to transferrin or can be phagocytosed by macrophages, enabling iron to be incorporated into the ferritin store. Possible adverse effects are: with i.m. injection, persistent pain at the injection site and skin discoloration; with i.v. injection, flushing, hypotension, anaphylactic shock.
|
|
Iron Compounds |
143 |
|
A. Iron: possible routes of administration and fate in the organism |
|
|
||
|
Fe(III) salts nonabsorbable |
|
|
|
Oral |
|
|
|
|
intake |
Fe(II) salts |
|
|
|
|
Heme-Fe |
|
|
|
|
|
Fe(III) |
|
|
Absorption |
|
Fe(III) |
|
|
Duodenum |
|
|
|
|
|
|
|
|
|
upper jejunum |
|
|
|
|
|
|
Ferritin |
|
|
|
|
Parenteral |
|
|
Transport |
|
administration |
|
|
Fe(III) |
Fe(III) |
|
|
|
Plasma |
|
|
||
|
|
|
|
|
|
Transferrin |
|
|
|
|
|
i.v. |
i.m. |
|
Uptake into |
Hemoglobin |
|
|
|
erythroblast |
|
Fe(III) complexes |
|
|
|
|
|
|
|
bone marrow |
|
|
|
|
|
|
Fe(III) |
|
|
Erythrocyte |
|
Ferritin |
|
|
|
|
|
|
|
blood |
|
|
|
|
|
|
Hemosiderin |
|
|
|
|
= aggregated |
|
|
|
|
ferritin |
|
|
Loss through |
|
Uptake into macrophages |
|
|
bleeding |
|
|
||
|
spleen, liver, bone marrow |
|
||
|
|
|
||
