
KAPLAN_USMLE_STEP_1_LECTURE_NOTES_2018_BIOCHEMISTRY_and_GENETICS
.pdf
Part I ● Biochemistry
•γ-Carboxylation: produces Ca2+ binding sites
•Prenylation: addition of farnesyl or geranylgeranyl lipid groups to certain membrane-associated proteins
POSTTRANSLATIONAL MODIFICATIONS OF COLLAGEN
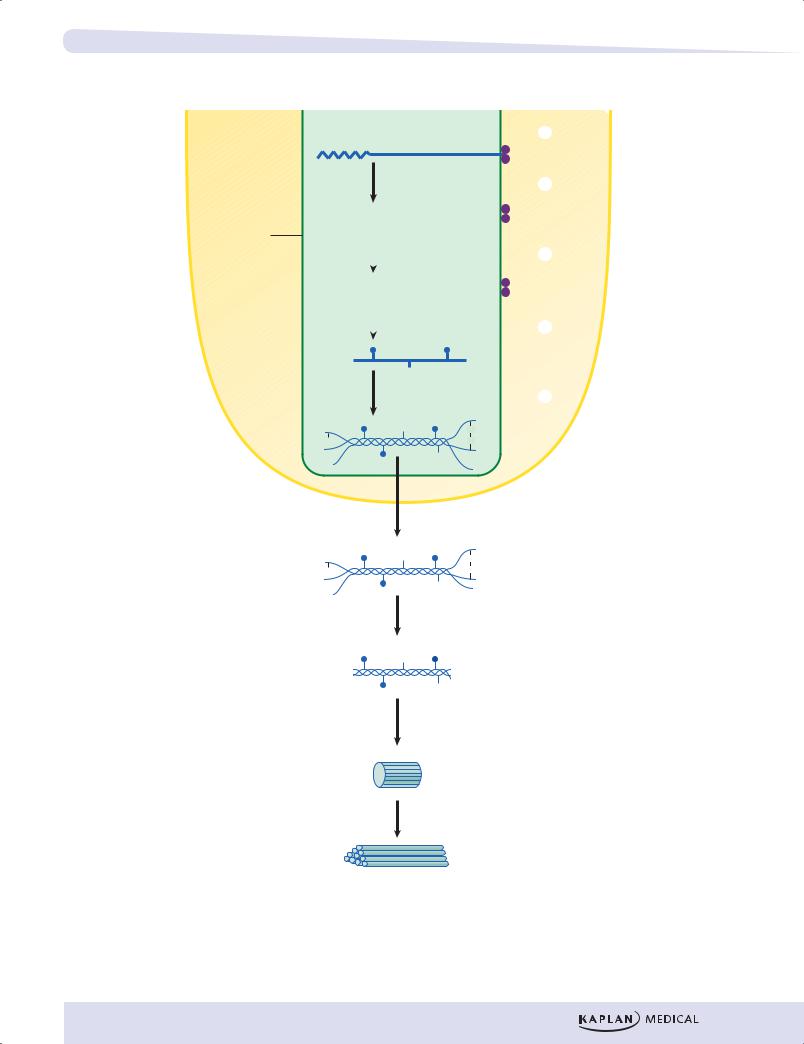
Collagen is an example of a protein that undergoes several important coand posttranslational modifications. It has a somewhat unique primary structure in that much of its length is composed of a repeating tripeptide Gly-X-Y-Gly-X-Y- etc. Hydroxyproline is an amino acid unique to collagen. The hydroxyproline is produced by hydroxylation of prolyl residues at the Y positions in procollagen chains as they pass through the RER.
1.Prepro-α chains containing a hydrophobic signal sequence are synthesized by ribosomes attached to the RER.
2.The hydrophobic signal sequence is removed by signal peptidase in the RER to form pro-α chains.
3.Selected prolines and lysines are hydroxylated by prolyl and lysyl hydroxylases. These enzymes, located in the RER, require ascorbate (vitamin C), deficiency of which produces scurvy.
4.Selected hydroxylysines are glycosylated.
5.Three pro-α chains assemble to form a triple helical structure (procollagen), which can now be transferred to the Golgi. Modification of oligosaccharide continues in the Golgi.
6.Procollagen is secreted from the cell.
7.The propeptides are cleaved from the ends of procollagen by proteases to form collagen molecules (also called tropocollagen).
8.Collagen molecules assemble into fibrils. Cross-linking involves lysyl oxidase, an enzyme that requires O2 and copper.
9.Fibrils aggregate and cross-link to form collagen fibers.
Table I-4-3. Collagen
|
Collagen |
|
|
Characteristics |
|
|
Tissue |
|
|
Associated Diseases |
|
|
Type |
|
|
|
|
|
Distribution |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
I |
|
Bundles of fibers |
|
Bone, skin, |
|
Osteogenesis imperfecta |
||||
|
|
|
|
High tensile strength |
|
tendons |
|
Ehlers-Danlos (various) |
|||
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
II |
|
Thin fibrils |
|
Cartilage |
---------- |
|
||||
|
|
|
|
Structural |
|
Vitreous humor |
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
III |
|
Thin fibrils |
|
Blood vessels |
|
Ehlers-Danlos |
||||
|
|
|
|
Pliable |
|
Granulation |
|
Type IV |
|||
|
|
|
|
|
|
|
tissue |
|
Keloid formation |
||
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
IV |
Amorphous |
Basement |
Goodpasture syndrome |
|||||||
|
|
|
|
|
|
membranes |
Alport disease |
||||
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
64
Chapter 4 ● The Genetic Code, Mutations, and Translation
Rough
Endoplasmic
Reticulum
(RER)
Synthesis of prepro-α chain with hydrophobic signal sequence
Removal of signal sequence by signal peptidase
Pro-α chain |
|
|
|
|
|
|
|
Hydroxylation of |
|
|
|||
|
|
|
|
|||
|
|
selected prolines and |
||||
|
|
lysines (vitamin C) |
||||
|
|
OH |
OH |
|||
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OH |
|
|
|
|
|
|
|
|||
|
|
Glycosylation of |
|
|
||
|
|
selected |
|
|
||
|
|
hydroxylysines |
|
|
||
Cytoplasm
|
OH |
|
|
Triple helix |
|
|
|
formation |
|
|
|
|
OH |
s |
|
s |
|
s |
|
s |
OH |
|
|
|
|
|
|
Procollagen secreted |
Secretion from cell |
|
|
(procollagen secreted) |
|||
s |
OH |
s |
|
|
s |
|
|
s |
OH |
|
|
|
|
|
|
|
Cleavage of propeptides |
||
|
OH |
Collagen |
|
|
OH |
(tropocollagen) |
|
|
|
|
|
|
Assembly into fibrils |
|
|
|
Stabilized by |
|
|
|
lysyl oxidase (Cu+) |
|
|
|
Aggregation to form |
|
|
|
a collagen fiber |
|
|
Figure I-4-9. Synthesis of Collagen
P
l
a
s
e n a r b m e M ma
65
Part I ● Biochemistry
Clinical Correlate
Ehlers-Danlos (ED) syndromes represent a collection of defects in the normal synthesis and processing of collagen. Like osteogenesis imperfecta, these syndromes are a result of locus heterogeneity in which defects in several different genes (loci) can result in similar symptoms.
ED Type IV, the vascular type, is an autosomal dominant disease caused by mutations in the gene for type-3 procollagen. Characteristic features include thin, translucent skin; arterial, intestinal, or uterine rupture; and easy bruising.
Also see Section II, Chapter 1; Locus Heterogeneity.
There are several important diseases associated with defective collagen production.
Table I-4-4. Disorders of Collagen Biosynthesis
|
Disease |
|
|
Defect |
|
|
Major Symptoms |
|
|
|
|
|
|
|
|
|
|
|
Scurvy |
|
Deficient hydroxylation |
|
Petechiae, ecchymoses |
|||
|
|
|
|
secondary to ascorbate |
|
Loose teeth, bleeding gums |
||
|
|
|
|
deficiency |
|
Poor wound healing |
||
|
|
|
|
|
|
|
Poor bone development |
|
|
|
|
|
|
|
|
|
|
|
Osteogenesis |
|
Mutations in collagen |
|
Skeletal deformities |
|||
|
imperfecta |
|
genes |
|
Fractures, blue sclera |
|||
|
|
|
|
|
|
|
|
|
|
Ehlers-Danlos |
|
Mutations in collagen |
|
Hyperextensible, fragile skin |
|||
|
syndromes |
|
genes and proline and |
|
Hypermobile joints, |
|||
|
|
|
|
lysyl hydroxylases |
|
dislocations, varicose veins, |
||
|
|
|
|
|
|
|
ecchymoses, arterial, |
|
|
|
|
|
|
|
|
intestinal ruptures |
|
|
|
|
|
|
|
|
|
|
|
Menkes disease |
|
Deficient cross-linking |
|
Depigmented (steely) hair |
|||
|
|
|
|
secondary to functional |
|
Arterial tortuosity, rupture |
||
|
|
|
|
copper deficiency |
|
Cerebral degeneration |
||
|
|
|
|
|
|
|
Osteoporosis, anemia |
|
|
|
|
|
|
|
|
|
|
Recall Question
Vitreous humor is formed from which type of collagen?
A.Type I
B.Type II
C.Type III
D.Type IV
Answer: B
66
Chapter 4 ● The Genetic Code, Mutations, and Translation
Menkes Disease
A 4-month-old infant who failed to grow and appeared to be mentally retarded was brought to the clinic for testing. The physician noted that the infant had abnormally kinky and hypopigmented hair. An arteriogram showed elongation and tortuosity of the major arteries. Additional tests revealed bladder diverticula and subdural hematomas. A blood test showed that the infant had low serum ceruloplasmin and only 10% of normal serum copper levels.
This infant has Menkes disease, which is also known as Ehlers-Danlos syndrome type IX (kinky hair syndrome). It is an X-linked recessive disease that has an incidence of 1/100,000 newborns. Common with Ehlers-Danlos diseases, Menkes disease has a symptomology due, in part, to weak collagen.
The disease is caused by mutations in the gene ATP7A, which encodes an ATP-dependent copper efflux protein in the intestine. Copper can be absorbed into the mucosal cell, but it cannot be transported into the bloodstream. Consequently, an affected individual will have severe copper deficiency and all copper-requiring enzymes will be adversely affected. Lysyl oxidase requires copper and plays a direct role in collagen formation by catalyzing the cross-linking of collagen fibrils. A deficiency in the activity of this enzyme and other copper-dependent enzymes would be directly responsible for the described symptoms in this infant.
67
Part I ● Biochemistry
Table I-4-5. Important Points About the Genetic Code, Mutations, and Translation
|
|
Prokaryotic |
|
|
Eukaryotic |
|
|
|
|
|
|
|
|
Genetic code |
|
Start: AUG (also codes for Met) |
|
|
|
|
|
|
Stop: UAG, UGA, UAA |
|
|
|
|
|
|
Unambiguous (1 codon = 1 amino acid) |
|
|
|
|
|
|
Redundant (1 amino acid >1 codon); often differ at base 3 |
||||
|
|
|
|
|
|
|
Mutations |
|
Point mutations: silent, missense, nonsense |
||||
|
|
Frameshift (delete 1 or 2 nucleotides; not multiple of 3) |
||||
|
|
Large segment deletion |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mutation in splice site |
|
|
|
|
|
|
Trinucleotide repeat expansion |
|
|
|
|
|
|
|
|
Amino acid |
|
Aminoacyl-tRNA synthetase: two high-energy bonds (ATP) to link amino acid to tRNA |
||||
activation |
|
|
|
|
|
|
|
|
|
|
|
|
|
Translation: |
|
30S subunit binds to Shine-Dalgarno |
|
40S subunit associates with 5′ cap on mRNA |
||
Initiation |
|
sequence on mRNA |
|
|
|
|
|
|
fMet–tRNAi binds to P site |
|
Met–tRNAi binds to P site |
||
|
|
GTP required |
|
GTP required |
||
|
|
|
|
|
|
|
Translation: |
|
Charged aminoacyl–tRNA binds to |
|
Charged aminoacyl–tRNA binds to A site (GTP) |
||
Elongation |
|
A site (GTP) |
|
|
|
|
|
|
Peptide bond forms (two high-energy |
|
28S rRNA is cut by Shiga and Shiga-like toxins |
||
|
|
bonds from amino acid activation) |
|
removing an adenine residue. Prevents protein |
||
|
|
|
|
|
synthesis. |
|
|
|
|
|
|
Peptide bond forms (two high-energy bonds from |
|
|
|
|
|
|
amino acid activation) |
|
|
|
Peptidyl transferase (50S subunit) |
|
Peptidyl transferase (60S subunit) |
||
|
|
Translocation: GTP required |
|
Translocation: GTP required |
||
|
|
|
|
|
eEF-2 inhibited by Diphtheria and Pseudomonas |
|
|
|
|
|
|
toxins |
|
|
|
|
|
|
|
|
Termination |
|
Release of protein; protein synthesized N to C |
||||
|
|
|
|
|
|
|
Protein targeting |
|
|
|
|
Secreted or membrane proteins: N-terminal |
|
|
|
|
|
|
hydrophobic signal sequence |
|
|
|
|
|
|
Lysosomal enzymes: phosphorylation of |
|
|
|
|
|
|
mannose by phosphotransferase in Golgi |
|
|
|
|
|
|
I-cell disease |
|
|
|
|
|
|
|
|
Other important |
|
|
|
|
Scurvy (prolyl hydroxylase, Vit C) |
|
disease |
|
|
|
|
Menke Disease (Cu deficiency, lysyl oxidase) |
|
associations |
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
68
Chapter 4 ● The Genetic Code, Mutations, and Translation
Review Questions
Select the ONE best answer.
1.In the genetic code of human nuclear DNA, one of the codons specifying the amino acid tyrosine is UAC. Another codon specifying this same amino acid is
A.AAC
B.UAG
C.UCC
D.AUG
E.UAU
Items 2 and 3
A.ATGCAA...→ ATGTAA
B.ATGAAA...→ GTGAAA
C.TATAAG...→ TCTAAG
D.CTTAAG...→ GTTAAG
E.ATGAAT...→ ATGCAT
The options above represent mutations in the DNA with base changes indicated in boldface type. For each mutation described in the questions below, choose the most closely related sequence change in the options above.
2.Nonsense mutation
3.Mutation decreasing the initiation of transcription
4.Accumulation of heme in reticulocytes can regulate globin synthesis by indirectly inactivating eIF-2. Which of the following steps is most directly affected by this mechanism?
A.Attachment of spliceosomes to pre-mRNA
B.Attachment of the ribosome to the endoplasmic reticulum
C.Met-tRNAmet binding to the P-site
D.Translocation of mRNA on the ribosome
E.Attachment of RNA polymerase II to the promoter
5.A nasopharyngeal swab obtained from a 4-month-old infant with rhinitis and paroxysmal coughing tested positive upon culture for Bordetella pertussis. He was admitted to the hospital for therapy with an antibiotic that inhibits the translocation of the 70S ribosomes on the mRNA. This patient was most likely treated with
A.erythromycin
B.tetracycline
C.chloramphenicol
D.rifamycin
E.levofloxacin
69
Part I ● Biochemistry
6.A 25-month-old Caucasian girl has coarse facial features and gingival hyperplasia and, at 2 months of age, began developing multiple, progressive symptoms of mental retardation, joint contractures, hepatomegaly, and cardiomegaly. Levels of lysosomal enzymes are elevated in her serum, and fibroblasts show phase-dense inclusions in the cytoplasm. Which of the following enzyme deficiencies is most consistent with these observations?
A.Golgi-associated phosphotransferase
B.Lysosomal α-1,4-glucosidase
C.Endoplasmic reticulum–associated signal peptidase
D.Cytoplasmic α-1,4-phosphorylase
E.Lysosomal hexosaminidase A
7.Parahemophilia is an autosomal recessive bleeding disorder characterized by a reduced plasma concentration of the Factor V blood coagulation protein. Deficiency arises from a 12 base-pair deletion in the Factor V gene that impairs the secretion of Factor V by hepatocytes and results in an abnormal accumulation of immunoreactive Factor V antigen in the cytoplasm. In which region of the Factor V gene would this mutation most likely be located?
A.5′ Untranslated region
B.First exon
C.Middle intron
D.Last exon
E.3′ Untranslated region
8.Collagen, the most abundant protein in the human body, is present in varying amounts in many tissues. If one wished to compare the collagen content of several tissues, one could measure their content of
A.glycine
B.proline
C.hydroxyproline
D.cysteine
E.lysine
9.A 6-month-old infant is seen in the emergency room with a fractured rib and subdural hematoma. The child’s hair is thin, colorless, and tangled. His serum copper level is 5.5 nM (normal for age, 11–12 nM). Developmental delay is prominent. A deficiency of which enzyme activity most closely relates to these symptoms?
A.Lysyl oxidase
B.Prolyl hydroxylase
C.γ-Glutamyl carboxylase
D.Phosphotransferase in Golgi
E.α-1,4-glucosidase
70
Chapter 4 ● The Genetic Code, Mutations, and Translation
10.Respiratory tract infections caused by Pseudomonas aeruginosa are associated with the secretion of exotoxin A by this organism. What effect will this toxin most likely have on eukaryotic cells?
A.Stimulation of nitric oxide (NO) synthesis
B.ADP-ribosylation of a Gs protein
C.ADP-ribosylation of eEF-2
D.ADP-ribosylation of a Gi protein
E.Stimulation of histamine release
11.A 4-year-old toddler with cystic fibrosis (CF) is seen by his physician for an upper respiratory infection with Pseudomonas aeruginosa. He is started on oral ciprofloxacin and is referred to a CF center as a potential candidate for gene therapy. Prior genetic testing of the patient identified the mutation causing CF as a 3-base-pair deletion in exon 10 of the CF gene. The nucleotide sequences of codons 506–511 in this region of the normal and mutant alleles are compared below.
Codon Number |
506 |
507 |
508 |
509 |
510 |
511 |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Normal Gene |
ATC |
ATC |
TTT |
GGT |
GTT |
TCC |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mutant Gene |
ATC |
AT• |
••T |
GGT |
GTT |
TCC |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
3-base |
|
|
|
|||
|
|
deletion |
|
|
|
|||
71
Part I ● Biochemistry
What effect will this patient’s mutation have on the amino acid sequence of the protein encoded by the CF gene?
|
U |
|
|
C |
|
A |
|
G |
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
UUU |
}Phe |
UCU |
|
|
UAU |
}Tyr |
UGU |
|
|
|
U |
|||
U |
UUC |
UCC |
|
Ser |
UAC |
UGC}Cys |
C |
||||||||
|
UUA |
|
Leu |
UCA |
} |
UAA |
} |
Stop |
UGA |
|
Stop |
A |
|||
|
UUG} |
|
|
UCG |
|
UAG |
|
UGG |
Trp |
G |
|||||
|
CUU |
|
|
|
CCU |
|
|
CAU |
|
|
CGU |
|
|
|
U |
C |
CUC |
|
|
Leu |
CCC |
|
Pro |
CAC} His |
CGC |
|
|
Arg |
C |
||
CUA |
|
|
CCA |
|
CAA |
|
|
CGA |
|
|
A |
||||
|
} |
|
|
|
|
|
|
|
|||||||
|
CUG |
CCG} |
|
CAG |
} |
Gln |
CGG} |
|
G |
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
AUU |
|
|
|
ACU |
|
|
AAU |
|
|
AGU |
}Ser |
U |
||
|
AUC |
|
|
lle |
ACC |
|
Thr |
AAC}Asn |
AGC |
C |
|||||
A |
AUA} |
|
ACA |
|
AAA |
|
|
AGA |
}Arg |
A |
|||||
|
|
|
|
|
|||||||||||
|
AUG |
|
Met |
ACG} |
|
AAG}Lys |
AGG |
G |
|||||||
|
GUU |
|
|
|
GCU |
|
GAU |
|
|
GGU |
|
|
U |
||
G |
GUC |
|
|
Val |
GCC |
Ala |
GAC} Asp |
GGC |
|
Gly |
C |
||||
GUA |
|
|
GCA |
|
GAA |
|
|
GGA |
|
|
|||||
|
GUG} |
GCG} |
GAG} Glu |
GGG |
} |
A |
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
G |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A.Deletion of a phenylalanine residue with no change in C-terminal sequence
B.Deletion of a leucine residue causing a change in the C-terminal sequence
C.Deletion of a phenylalanine residue causing a change in the C-terminal sequence
D.Deletion of a leucine residue with no change in C-terminal sequence
12.A 10-year-old boy with severe progressive skin ulceration, decreased resistance to infection, and impaired cognitive ability has been diagnosed with a genetic deficiency of the enzyme prolidase. Mutation analysis has identified a single base substitution at the 3’ end of intron 6 of the mutant allele as well as deletion of a 45-base exon (exon 7) in the prolidase cDNA. Which type of gene mutation was most likely inherited by this boy?
A.Frameshift mutation
B.In-frame mutation
C.Missense mutation
D.Nonsense mutation
E.Splice site mutation
72
Chapter 4 ● The Genetic Code, Mutations, and Translation
Answers
1.Answer: E. Because of wobble codons for the same amino acid often differ in the third base. Option B would be acceptable, except that it is a stop codon.
2.Answer: A. The sequence now contains TAA which will be transcribed to UAA in the mRNA.
3.Answer: C. The transcription promoter TATA has been changed to TCTA. Don’t choose the distractor B. The question is not about translation.
4.Answer: C. eIF-2 designates a protein factor of the initiation phase in eukaryotic translation. The only event listed that would occur during this phase is placement of initiator tRNA in the P-site.
5.Answer: A. Erythromycin is the antibiotic of choice for pertussis. It inhibits translocation.
6.Answer: A. Characteristic symptoms of I-cell disease. Note release of lysosomal enzymes into serum, which would not be seen in the other deficiencies.
7.Answer: B. Decreased factor V secretion and a corresponding accumulation of cytoplasmic antigen suggest a defect in the translocation of the nascent protein to the endoplasmic reticulum. This implies a mutation in the N-terminal amino acid signal sequence required for targeting to the ER and encoded by the first exon of the gene.
8.Answer: C. Hydroxyproline is found uniquely in collagen. Although collagen is also rich in glycine, many other proteins contain significant amounts of glycine.
9.Answer: A. The child has Menkes disease, in which cellular copper transport is abnormal and produces a functional copper deficiency. Lysyl oxidase in collagen metabolism requires copper. His fragile bones and blood vessels result from weak, poorly crosslinked connective tissue.
10.Answer: C. Pseudomonas and diphtheria toxins inhibit eEF-2, the translocation factor in eukaryotic translation.
11.Answer: A. Deletion of CTT results only in the loss of phe 508; ile 507 and the C-terminal sequence are unaltered because ATC and ATT both code for ile (the coding sequence is unchanged).
12.Answer: E. A base substitution at an intron-exon junction, which leads to the deletion of an entire exon is indicative of a splice site mutation.
73
