
SELF-CHECK QUESTIONS
1.What is the purpose of writing an introduction for the author?
2.What is the purpose of writing an introduction for the reader?
3.How many stages are typically included in an introduction?
4.What functions do they perform?
5.What is the order of information elements in Stage 1?
6.What is reviewing of previous works in Stage 2 aimed at?
7.Are in-text references acceptable in an introduction?
8.What types of ordering citations can be employed?
9.What signal words are used to indicate Stage 3?
10.What information element is directly derived from Stage 3?
11.What verb tenses are conventionally used in introductions?
12.What articles are used with generic and specific noun phrases?
U n i t 4. MATERIALS AND METHODS
"The key to a successful Methods section is to include the right amount of detail –
too much, and it begins to sound like a laboratory manual; too little, and no one can repeat what was done."
THE AIMS OF THE UNIT:
to make you think about the purposes of writing the Materials and Methods section;
to provide insight into typical elements of information included in the Materials and Methods section and the order they follow;
to analyse language features of the Materials and Methods section;
to provide practice in identifying and reconstructing different information elements which the Materials and Methods section contains;
to practise in writing the Materials and Methods section of your own.
STARTING POINT
After the Introduction, the second major section of the experimental research paper is Materials and Methods. This combined title indicates that researchers generally describe these two aspects together when they write up their research.
The purpose of the Materials and Methods section is to describe in detail how the study/experiment was carried out and also to clarify the rationale for the procedure. In science, it's not sufficient merely to design and carry out an experiment. Ultimately, others must be able to verify your findings, so your experiment must be reproducible, to the extent that other researchers can follow the same procedure and obtain the same results.
67
In the Materials and Methods section, you can write that you recorded the results, or how you recorded the results (e.g., in a table), but you shouldn't write what the results were – not yet. Here, you're merely stating exactly how you went about testing your hypothesis.
In the following example from the field of Powder Technology, notice the elements that have been included under Materials and Methods.
EFFECT OF THE SPRAY-DRYING PROCESS ON THE PROPERTIES OF COATED FILMS IN FLUIDIZED BED GRANULAR COATERS1
MATERIALS AND METHODS
2.1.
The following three types of coater with different spraying/evaporation properties were used.
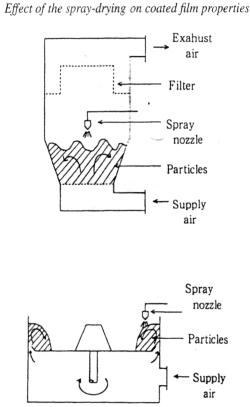
2.1.1. Conventional fluidized bed coater [4]. Figure 1 shows a schematic view of a conventional fluidized bed coater (Freund Industrial; FLO-5: bottom screen diameter 230 mm).
The coating liquid is sprayed downward onto particles as it is fluidized by air flow from below. This spray method is commonly referred to as the top-spray method. The distance from spray nozzle to the surface of the particles is about 10-20 cm.
|
|
2.1.2. |
|
Centrifugal |
|||
|
tumbling |
|
coater |
|
[4 – 6]. |
||
|
Figure 2 shows a schematic |
||||||
|
view |
of |
a |
centrifugal |
|||
|
tumbling |
|
coater |
(Freund |
|||
Figure 1. Schematic view of a fluidized bed |
Industrial; |
CF-360: |
rotor |
||||
diameter |
|
360 mm). |
The |
||||
coater. |
coating |
liquid |
is |
sprayed |
|||
|
slantdownward |
|
|
onto |
|||
|
particles. This spray method |
||||||
|
is |
called |
|
diagonal-top- |
|||
|
spraying in this article. There |
||||||
|
is small amount of air flow |
||||||
|
from below through a slit |
||||||
|
between the inner wall of the |
||||||
|
casing and the outer surface |
||||||
|
of the rotary disk edge. The |
||||||
|
distance |
from |
the |
|
spray |
||
|
nozzle to the surface of the |
||||||
Figure 2. Schematic view of a CF coater. |
particles is about 10 cm. |
||||||
68
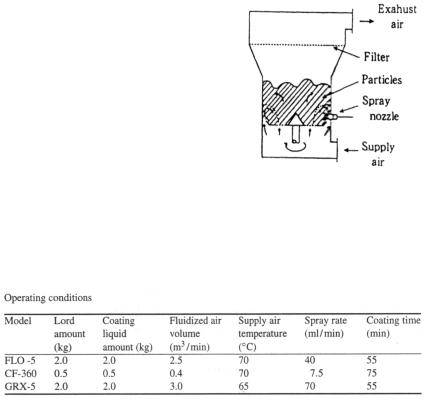
2.1.3. Centrifugal rotary fluidized bed coater [7 – 11]. Figure 3 shows a schematic view of a centrifugal rotary fluidized bed coater (Freund Industrial; GRX-5: rotor diameter 260 mm). The spray nozzle is equipped on the sidewall near the rotary disk. The coating liquid is sprayed horizontally onto particles. This spray method is commonly referred to as the side-spray method. The spray nozzle tip nearly touches the surface of the particles.
2.2.
2.2.1. Materials. Nonpareil-103 (diameter 500-710 µm; Freund Industrial)
coated with blue dye (brilliant blue) was used as core material. The formulation of coating liquid was as follows: 4% ethyl cellulose (N-10-F; Shin-Etsu Chemical) as a polymer and a mixture of ethanol: water at a ratio of 8 : 2 used as a solvent.
2.2.2. Preparation of coated particles. In the coating process, agglomeration of particles occurs when the surface of particles are excessively wet [7, 12, 13]. Therefore, the supply air temperature was determined to keep the material temperature at 42-43 °C during the coating process. Since the drying capability of CF-360 is lower, materials loading volume in CF-360 is reduced to 25% of the loaded volume for other coaters. Table 1 shows the main operating conditions.
2.3.
2.3.1. Adhesion efficiency of ethyl cellulose. The adhesion efficiency of ethyl cellulose was evaluated by the following measures:
(i)500 mg of coated particles was dissolved in a mixture solvent of ethanol: water at a ratio of 8 : 2 and content of blue dye in this solvent was measured by absorbance.
(ii)The content of core particles was calculated from the content of
blue dye.
(iii)The content of ethyl cellulose was calculated from the content of core particles.
69
2.3.2.Dissolution test. A dissolution test was carried out according to the paddle method of JP XIV (37°, 100 r.p.m.). A sample of 3 g of coated particles was placed in a test vessel containing 900 ml of 1st liquid of JP XIV and the concentration of blue dye in the solution was determined by spectrophotometery.
2.3.3.Scanning electron microscopy. The surface condition of coated particles was observed by a scanning electron microscope (ABT-55; Topcon).
Notes:
spray-drying process – процесс сушки распылением coated films – покровные плёнки
fluidized bed granular coaters – псевдоожиженные гранулированные установки для нанесения покрытий
spraying – распыление, орошение evaporation – напыление
liquid – жидкость
downward – спускающийся, нисходящий, направленный книзу nozzle – распылитель, распылитель форсунки, сопло, наконечник,
насадок, патрубок
rotor – ротор, рабочее колесо
tumbling coater – галтовочная установка для нанесения покрытий slantdownward – спускающийся, нисходящий, направленный книзу
наклонно, под углом solvent – растворитель
agglomeration of particles – накапливание; концентрация,
аккумуляция, сосредоточение частиц
loaded – нагруженный, насыщенный, наполненный
the adhesion efficiency – эффективность адгезии, прилипания, сцепления
blue dye – голубая окраска paddle method – лопастной метод
test vessel – резервуар, сосуд, баллон, камера для проведения теста
In this abstract we can outline:
materials (laboratory equipment) and methods (procedure steps)
2.1.1.Conventional fluidized bed coater
2.1.2.Centrifugal tumbling coater
2.1.3.Centrifugal rotary fluidized bed coater
materials (substances) 2.2.1. Materials
methods (procedure steps)
2.2.2. Preparation of coated particles
70
methods (procedure steps) and materials (laboratory equipment, test) 2.3.1.Adhesion efficiency of ethyl cellulose
2.3.2.Dissolution test.
2.3.3.Scanning electron microscopy
FOCUS ON STRUCTURING INFORMATION
1. Materials and Methods is a relatively formulaic section in that there is a clearly marked out structure to follow.
Elements of information that can be included in the Materials and Methods section:
∙Overview of the Experiment
∙Population/Sample
∙Location
∙Restrictions/Limiting Conditions
∙Sampling Technique
∙Procedures*
∙Materials*
∙Variables
∙Statistical Treatment
(* always included)
By materials we mean any items used to carry out a research project. They may fall into any of the following categories:
laboratory equipment field equipment
human or animal subjects materials natural substances
fabricated materials
surveys, questionnaires and tests computer models
mathematical models
TASK 4.1. Read and translate the following abstract from a paper written in the field of Powder Technology. Identify the information elements and answer the following questions:
a.What elements other than procedures and materials did the author include in this section?
b.Why do you think the author chose to order the elements in this way?
c.Did you find this procedural description clear and easy to understand?
d.What type of material is described in this example, based on the categories listed in the previous scheme?
71
A METHOD FOR EVALUATION OF THE COMPONENT UNIFORMITY OF A POWDER MIXTURE BY MICRO FOURIER TRANSFORM INFRARED SPECTROMETRY2
2.1. Sample
Three kinds of commercial silica powders (specific surface area: 200 m2/g, Hokkoukakgaku Ltd.; 79 m2/g, Yoneyamakagaku Ltd.; and 1.4 m2/g, Katayamakagaku Ltd.) were each mixed with 4 g of commercial zirconia powder (specific surface area 26 m2/g, Daiichikigenso Ltd.). These mixtures were named M1, M2 and M3, respectively. The silica and zirconia powder were put into bottles (polystyrene, 50 ml) with three polymethylmethacrylate balls and vibrated for 30 min using a mixer mill (model 8000; Spex Ltd.). A silicon alkoxide and zirconium alkoxide mixture was hydrolyzed and heated at 600 °C, and a silica and zirconia mixture (the component ratio nearly same to the above mixture) was obtained. This mixture was named A1 .
2.2. Test apparatus and measuring condition
The apparatus used for the test was a micro-FTIR spectrometer (Janssen; Jasco Ltd.). About 20 mg of the sample was put into a 5 mm die and pressed at 19.6 MPa.
Infrared radiation, focused by a x 16 Cassegrain mirror, was irradiated on to the sample surface, and the reflection was collected by the same mirror and detected by a mercury-cadmium-tellurium (MCT) semiconductor detector following passage through an aperture to limit the measuring spot size. An aluminum mirror was used for a reference sample. The measurement spot size was changed from 250 x 250, 80 x 80 and 25 x 25 µm by changing the aperture size. Ten spectra were measured at different locations in the sample pellet for each spot size.
The chemical analysis of silicon and zirconium in the samples was measured by inductively coupled plasma (ICP) emission spectrometry (wavelength: Si I 251.611 nm and Zr II 343.823 nm, respectively, model ICAP 1000 s; Nihon Jurrel Assu Ltd.). The sample solution was prepared by decomposing 100 mg of the mixture with 5 ml of hydrochloric acid and hydrofluoric acid in a PTFE vessel for 3 h at 170 °С and diluting to 100 ml with distilled water. The concentration ratio between silica and zirconia was calculated from the concentration of Si and Zr in the solution.
Notes:
uniformity – однородность, единообразие, равномерность (смешивания) powder mixture – порошковая смесь
micro Fourier – микро коэффициент Фурье
infrared spectrometry – инфракрасная спектрометрия commercial silica powders – технический кварцевый порошок
commercial zirconia powder – технический циркониевый порошок polymethylmethacrylate balls – полиметилметакрилатовые шары mixer mill – смешивающая мельница
alkoxide – алкоксилированный hydrolyzed – гидролизованный
72
component ratio – пропорция составных частей sample – образец
die – пресс– форма
Cassegrain mirror – ( зеркальная) система Кассегрена (кассегреновское зеркало)
to irradiate – испускать лучи to detect – обнаруживать
a mercury-cadmium-tellurium (MCT) semiconductor detector – ртутно-
кадмиево-теллуровый (РКТ) полупроводниковый детектор an aperture – диафрагма
sample pellet – образец гранул
inductively coupled plasma – индуктивно связанная плазма emission spectrometry – эмиссионная спектрометрия
by decomposing – путем разложения hydrochloric acid – соляная кислота hydrofluoric acid – плавиковая кислота vessel – резервуар, сосуд, баллон, камера
TASK 4.2. Read the following example of Materials and Methods section from the field of Nanotechnology. Identify the information elements you find in each sentence. (NOTE: Some sentences may contain more than one element.)
CARBON NANOTUBES SYNTHESIZED BY NI-ASSISTED ATMOSPHERIC PRESSURE THERMAL CHEMICAL VAPOR DEPOSITION
1Ni films of 10-40 nm were deposited on Si(100) substrates using the dc magnetron sputtering method. 2The substrates with Ni films were placed in a 7.5-cm-diameter resistance heated quartz tube furnace at room temperature and were pumped down to less than ~10-3 Torr using a mechanical pump. 3The substrate was then heated to a synthesis temperature while introducing H2 gas for a reducing environment. 4The synthesis temperature was in the range 500-900 °C. 5When the temperature was stabilized, H2 or NH3 gas was introduced and then either C2H2 or NH3/C2H2 mixture gases followed. 6The gas pressure was ~ 1 atm and the growth time was varied in 2 h. 7At the end of the growth, the samples were furnace-cooled slowly in a hydrogen flowing environment.
8The morphologies of catalytic Ni films and CNTs were examined using SEM, high-resolution ТЕМ (HRTEM; model Philips CM20T/STEM), and Raman spectroscopy. 9In addition to the conventional ТЕМ observation of CNTs on microgrid, we also prepared specimens for cross-sectional observations to investigate the interface between the CNTs and the substrate. 10In order to examine the local composition in the cross section of the structures, energy dispersive x-ray (EDX) spectroscopy measurements were also made.
73
Notes:
vapor deposition – осаждения из паровой фазы
Ni films of 10-40 nm were deposited on Si(100) substrates – Ni пленки
10-40 нм наносились на Si (100)подложки sputtering method – метод распыления tube furnace – трубчатая печь
to pump down – нагнетать
reducing environment – восстановительная среда the synthesis temperature – температура синтеза growth time – время роста
the samples were furnace-cooled slowly in a hydrogen flowing environment – образцы медленно охлаждались в печи в водородной среде
the morphologies of catalytic Ni films – структура пленок с каталитическими Ni пленками
high-resolution – высокое разрешение
In addition to the conventional ТЕМ observation of CNTs on microgrid… – В добавление к традиционному наблюдению за УНТ с помощью просвечивающей электронной микроскопии (ТЕМ) на микросетке…
specimens for cross-sectional observations – образцы для наблюдения поперечного сечения
interface between the CNTs and the substrate – граница между УНТ и подложкой
the cross section of the structures – поперечное сечение структур
energy dispersive x-ray (EDX) spectroscopy measurements – замеры с помощью энергетической дисперсивной рентгеновской спектроскопии
ELEMENT
Sentence 1
Sentence 2
Sentence 3
Sentence 4
Sentence 5
Sentence 6
Sentence 7
Sentence 8
Sentence 9
Sentence 10
74
TASK 4.3. Surf the Internet, go to the library or address your scientific advisor in order to find an experimental research paper in your field. Locate the section corresponding to Materials and Methods, analyze it and answer the following questions.
a. Is the section in your report labeled "method"? If not, what it called?
b.Which of the elements from the list on pages 5 – 6 can you find in your example? In what order are they presented?
c.Read the part of your example that describes the procedure used in the study. Is it written clearly enough so that you can easily understand it, or not? Why do think so?
FOCUS ON LANGUAGE USE
1. The description of the steps you followed in conducting your study should be written clearly so that a reader in your field could accurately replicate your procedure. The best way to describe a procedure is step-by-step, or chronologycally. To make it, we mention dates and time, and we also use various links and connectives.
Time
In 2011, ...
During the 20th century, ...
Yesterday, ...
Twenty five years ago, ...
Sequence
before
Before he was offered a job as a lecturer, Before this, …
For the previous X years, … Prior to this, …
Previously, …
X years previously, …
he had finished his research.
after
When
As soon as he had finished his research, he was offered a job as a lecturer. After
On finishing his research, |
he was offered a job as a lecturer. |
After finishing his research, |
|
For the following X years, … |
|
X years later, … |
|
75
After …
Following this, …
When …
Subsequently, …
while
While he was doing his research, When doing his research,
While doing his research, During his research, During this period, … Throughout this period, …
…during which…
…throughout which…
he made an important discovery.
TASK 4.4. Scan the following abstract from a paper written in the field of Nanotechnology. Identify and underline the indicators of sequence you can find.
THE DETERMINING FACTORS FOR THE GROWTH MODE OF CARBON NANOTUBES IN THE CHEMICAL VAPOUR DEPOSITION PROCESS4
For synthesis of CNTs, thermal and PECVD methods have been used. The equipment employed is shown schematically in figure 1. The thermal CVD equipment is a resistance heated quartz tube furnace of 3 inch diameter with three-zone temperature control. In PECVD, the gases are supplied through a capacitively coupled shower type electrode at the top of the reactor. The gases used (C2H2, NH3, H2) are of 5 N purity except N2 (3N purity), and their flow was controlled by mass flow controllers. All the substrates used were degreased in trichloroethylene, acetone, and methanol, without etchcleaning of the surface, before sputter deposition and spin-coaling of the magnetic.
In PECVD syntheses, Si (100) wafer sputter deposited with Fe thin films (35 nm) was mounted on the heater plate in the chamber at room temperature. The specimens are usually of the size of 1 cm x 1 cm. After evacuation of the chamber to the mTorr range, hydrogen gas was introduced into the chamber while increasing the sample temperature. When the temperature had reached 700 °C, hydrogen gas was turned off and ammonia gas was turned on.
The plasma power was then turned on to 600 W for plasma treatment of the metal films. For CNT growth, NH3 and C2H2 gas mixture was introduced while maintaining the chamber pressure at ~0.1 Torr. At the end of the growth, plasma power and gases were turned off, and nitrogen gas was introduced for cool-down of the samples. The CNTs were grown with varying plasma power to vary the plasma bombardment effect on the substrate, and subsequently the growth modes.
In thermal CVD syntheses on various substrates, magnetic fluid of Ее304 as a catalyst [11] was spin coated on a sapphire wafer, alumina deposited on
76
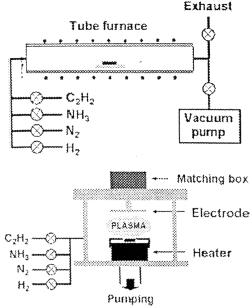
an Si wafer via atomic layer deposition (ALD), and the backside of an anodized aluminium oxide (AAO) membrane. The thermal CVD process was employed for CNT synthesis and is described elsewhere in detail [8, 12]. The substrates coated with magnetic fluid were mounted directly in a quartz tube furnace and the tube was evacuated.
Figure 1. Schematic diagrams of the equipment employed for thermal and plasma-enhanced CVD syntheses.
When the temperature reached 700 °C in vacuum, hydrogen gas was introduced into the furnace until atmospheric pressure was established in the furnace. When the growth temperature reached 900 °C, hydrogen gas was turned off and ammonia gas was introduced for 5 min for pretreatment of the catalyst metal films. After this pretreatment step, C2H2 gas was supplied for 10 min. The flow rates of NH3 and C2H2 were maintained at 60 and 30 seem, respectively. The working pressure was 1 atm during the growth of CNTs. All the substrates were coated with magnetic fluid by the spin coating method. The thickness of the magnetic fluids, and thus the size of the catalytic particles, on substrates was controlled by the rotation speed of the spin-coater. A good distribution of ~50 nm particles was obtained at 4000 rpm. The average growth rate on sapphire and ALD was ~1 mm min-1 and that on AAO was ~200 nm min-1 when the growth was carried out for 10 min. Note, however, that the calculated growth rates are not the real ones due to the incubation time for the growth initiation [8]. Due to the uniqueness of the growth mode on the AAO membrane, supplementary experiments to confirm the relevant growth mode
77
were carried out on AAO membranes. The morphology of the substrates and CNTs were examined by field emission scanning electron microscopy (FHSEM), high resolution transmission electron microscopy (HRTEM), and atomic force microscopy (AFM). Energy dispersive x-ray spectroscopy (EDX) measurement was employed in determining the iron content at the spots of interest.
Notes:
the growth mode of carbon nanotubes – выращивание углеродных нанотрубок
chemical vapour deposition process – процесс химического осаждения из паровой фазы
a resistance heated quartz tube furnace – кварцевая трубчатая печь сопротивления
capacitively coupled shower type electrode – емкостно-спаренный распылитель электрода
purity – чистота
All the substrates used were degreased in trichloroethylene... – Все использованные подложки обезжиривались в трихлорэтилене…
without etchcleaning of the surface, before sputter deposition and spincoaling of the magnetic – без травления поверхности для очистки, перед осаждением напыления и покрытием магнитных текучих сред
In PECVD syntheses, Si (100) wafer sputter deposited with Fe thin films (35 nm) was mounted on the heater plate in the chamber… – В синтезах ХОП, усиленных плазмой, Si (100) подложка, с напылением в виде тонких Fe пленок (35 нм), была установлена на пластину нагревателя в камере…
hydrogen gas was turned off and ammonia gas was turned on – убрали водородный газ и подали газообразный аммиак
nitrogen gas was introduced for cool-down of the samples –
газообразный азот был введен для охлаждения образцов
to vary the plasma bombardment effect on the substrate – варьировать эффект бомбардировки плазмы на подложке
In thermal CVD syntheses on various substrates, magnetic fluid of Ее3O4 as a catalyst [11] was spin coated on a sapphire wafer, alumina deposited on an
Si wafer via atomic layer deposition (ALD), and the backside of an anodized aluminium oxide (AAO) membrane. – В синтезе термического ХОП на различных подложках магнитный флюид Fe3O4 качестве катализатора [11] был покрыт методом центрифугирования на подложку сапфира, окись алюминия, осевшую на Si подложку путем осаждения атомного слоя и обратную сторону мембраны анодированного оксида алюминия.
The substrates coated with magnetic fluid… – Подложки, покрытые магнитным флюидом…
for pretreatment of the catalyst metal films – для предварительной обработки металлических пленок катализатора
78
the spin coating method – метод центрифугирования the spin-coater – центрифуга
The average growth rate on sapphire… – Средняя скорость роста на сапфире…
field emission – полевая эмиссия
spots of interest – интересующие участки
TASK 4.5. The Materials and Methods section from a research paper in the field of Food and Nutrition is given here with the paragraphs in scrambled order. Rearranged the paragraphs in a more conventional order, as you think the authors originally wrote them.
a.-------- It is important to note that the extract to be tested was added to each tube immediately before placing the tube into the spectrophotometer.
1.0ml of catecholase extract was pipetted into tube 2. Tube 2 was immediately inverted and placed in the spectrophotometer. The absorbance was read and recorded for time zero (t0), the ten minute mark (t10), and each minute in between. Tube 2 was removed from the spectrophotometer and the same measurements were taken for tube 3 and tube 4 using the same protocol.
b.-------- In preparing the catecholase extract, a potato was skinned, washed, and diced. 30.0 g of the diced potato and 150 ml of distilled water were added to a kitchen blender and blended for approximately two minutes. The resulting solution was filtered through four layers of cheese cloth. The extract was stored in a clean, capped container.
c.-------- Four individually labeled spectrophotometer tubes were prepared using different amounts of the following reagents: a buffer of pH 7, a 0.1% catechol substrate, and distilled water. The wavelength of the Spectronic 20 spectrophotometer was set at 540 nm. To calibrate the specrophotometer at zero absorbance, a blank control tube prepared with no catechol substrate and labeled "tube 1" was inverted and inserted into the spectrophotometer.
Notes:
spectrophotometer – спектрофотометр
1.0 ml of catecholase extract was pipetted into tube 2 – 1,0 мл экстракта катехола пипеткой поместили в пробирку 2
the absorbance – абсорбция
was skinned, washed, and diced – был почищен, промыт и нарезан capped container – ограниченный контейнер
a buffer – буфер
catechol substrate – катехол подложки
2. Remember that you're talking about an event which happened at a particular time in the past, and which has already ended by the time you start writing, so simple past tense will be appropriate in this section.
e.g.: "We added 5 g of the solid to the solution". or "5 g of the solid were added to the solution".
79

Sentences, that are not written in the past tense usually do not refer to the procedures used in the study being reported.
Very often scientific journals encouraged their writers to avoid using the first person ("I" or "we"), because the researchers themselves weren't personally important to the procedure in the experiment. To help keep personal references out of lab reports, scientific conventions also dictated that researchers should use passive voice, in which the subject of a sentence or clause doesn't perform the action described by the verb.
e.g.: Active: We heated the solution to 80 °C. (The subject, "w e," performs the action, heating.)
e.g.: Passive: The solution was heated to 80 °C. (The subject, "s olution," doesn't do the heating -- it is acted upon, not acting.)
Increasingly, especially in the social sciences, using first person and active voice is acceptable in scientific reports. Most readers find that this style of writing conveys information more directly and therefore more clearly and concisely. This rhetorical choice thus brings two scientific values into conflict: objectivity versus clarity. Since the scientific community hasn't reached a consensus about which style it prefers, you may want to ask your scientific advisor.
TASK 4.6. Read and translate the following abstract from a paper written in the field of Powder Technology. Pay attention to the use of verb tenses and voice.
PARTICLE FORMATION BY THE DILUTION METHOD USING
A MISCIBLE NON-SOLVENT5
A sulfur solution in ethanol was prepared and filtered through a 0.1 µm membrane filter to remove impurities. This solution was then diluted with ethanol to obtain the desired concentration. Sulfur particles were generated by mixing the sulfur solution with pure water, which is a miscible non-solvent for sulfur. Experiments were carried out with varying initial sulfur concentrations, and mass fractions of miscible non-solvent of 25, 50, 75 wt%. This method corresponds to case (c) mentioned above. The amount of solution after mixing is 8 x 10-3 kg. This will be referred to as the sulfur system.
A lead chloride solution in pure water was prepared and filtered through a 0.1 µm membrane filter. This solution was then diluted with pure water to obtain an initial monomer concentration of 4.0 x 10-3 kg/kg-solvent. Lead chloride particles were generated by mixing this solution with ethanol, which is a miscible non-solvent for lead chloride. Experiments were carried out with varying mass fractions of miscible non-solvent and keeping the initial monomer concentration constant. This mixing method corresponds to case (a) above. The amount of solution after mixing is 8 x 10-3 kg. It will be referred to as the lead chloride system.
When water and ethanol are mixed, the solution temperature increases by the heat of dissolution. The mixtures were cooled down to give a final solution temperature of 293 K.
80
For the measurement of the total particle concentration n*, a sample was taken from the test tube and placed onto a slide glass. The slide glass with the sample was then covered by another glass with a certain gap and observed by an optical microscope. Once the particles had deposited onto the slide glass by sedimentation, their concentration was measured by counting the number of particles existing in a known volume between the two slide glasses. The mean volume diameter dv of the generated particles was determined from scanning electron microscope micrographs.
Notes:
dilution method – метод разбавления miscible – допускающий смешивание non-solvent – нерастворитель
A sulfur solution in ethanol… – Раствор серы в этаноле… to dilute – разжижать, разбавлять
to remove impurities – удалять примеси mass fractions – массовые доли
lead chloride solution – раствор хлорида свинца monomer concentration – концентрация мономера dissolution – растворение, распад, разложение mixtures – смеси
slide glass – предметное стекло gap – отверстие
sedimentation – осаждение micrographs – микрофотографии
TASK 4.7. In the abstract below (from the field of Nanotechnology) put the verbs in the appropriate tense form.
TEMPERATURE EFFECT ON THE GROWTH OF CARBON NANOTUBES USING THERMAL CHEMICAL VAPOR DEPOSITION6
20 mm x 30 mm sized p-type Si (100) substrates with a resistivity of 15 cm ____thermally ____(oxidize). The thickness of silicon oxide (SiO2) layer _____ _____(estimate) approximately as 300 nm. A 30 nm-thick Fe film
_____thermally _____(deposit) on SiO2 layer using a thermal evaporator under a pressure of 10-6 Torr. The Fe-deposited substrates ____ ____(load) with face down direction on a quartz boat in quartz CVD reactor which was maintained in atmospheric pressure. The diameter of reactor tube ___(be) 550 mm. Argon (Ar) gas with a flow rate of 1000 sccm ____ ____ (supply) into the CVD reactor to prevent the oxidation of catalytic metal while raising the temperature. In order to form the catalytic particles in nanometer size, the substrates ____ ____
(pretreat) by ammonia (NH3) gas with a flow rate of 100 sccm for 20 min in the temperature range 750-950 °C. The CNTs ___ _____(grow) on the substrate using C2H2 with a flow rate of 30 sccm for 10 min at the same temperature of NH3 pretreatment in atmospheric pressure.
81
The CNTs ____ ____(examine) by SEM (Hitachi S-800, 30 kV) to measure the length, diameter, uniformity, and density. ТЕМ (Philips, CM20T, 200 kV) ___ ____ (use) to investigate the structure and crystallinity of CNTs. The CNTs ____ ____(separate) from the substrate and then ____(disperse) on a carbon ТЕМ microgrid. TGA (ТА instrument TGA 2050) ___ ___ ____
(use) for temperature-programmed oxidation at a constant heating rate of 10 °C/min using air, in order to measure the degree of crystalline perfection. The weight loss of CNTs ___ ____(record) as a function of temperature and time. A Raman spectrometer (Renishaw micro-Raman 2000) ____ also ____
(use) to identify the structure and the crystallinity of CNTs. The 632.8 nm line of a He-Ne laser ____ ____(use) for excitation.
Notes:
growth of carbon nanotubes using thermal chemical vapor deposition –
рост углеродных нанотрубок при химическом термическом осаждении из пара
to oxidize – окислять
oxide (SiO2) layer – окисный слой (SiO2) to deposit – наносить
thermal evaporator – термический испаритель
the Fe-deposited substrates – подложки с тонкой пленкой железа quartz boat – кварцевая лодочка
Argon (Ar) gas – аргоновый газ
the oxidation of catalytic metal – окисление каталитического металла to pretreat – предварительно обработать
uniformity – равномерность disperse – рассеивать microgrid – микрорешетка oxidation – окисление
crystalline perfection – кристаллографическое совершенство crystallinity – кристалличность
excitation – активизация
TASK 4.8. The following procedure description was taken from an article in the field of Powder Technology. It has been altered so that the writers of the article are mentioned as agents in each sentence. Rewrite the description in a depersonalized form.
PARTICLE MORPHOLOGY AND BATTERY PROPERTIES OF LITHIUM MANGANATE SYNTHESIZED BY ULTRASONIC SPRAY PYROLYSIS7
2.1. Starting materials
We used metal nitrate and acetate as starting materials. We used LiNO3 and Li(CH3COO ) as the Li source and we used Mn(N03)2 • 6H2O and Mn(CH3COO)2 • 4H2O as the Mn source, respectively. We weighed out LiN03
82
and Mn(NO3)2 • 6H2O or Li(CH3COO) and Mn(CH3COO)2 • 4H2O to attain a molar ratio of metal components (Li: Mn) of 2 : 1 and we dissolved them in double-distilled water to prepare aqueous solutions of 0.1 mol/dm3.
2.2. Preparation and characterization of LiМn2О4 powders
We generated the mist of starting solution prepared with an ultrasonic vibrator (1.6 MHz) and introduced it into quartz tube (diameter 38 mm x 2000 mm) in an electrical furnace with an air carrier (7 dm3/min). We dried the mist at 400 °C and then decomposed it at 900 °C. We collected as-prepared powders using a cyclone [12]. We determined the morphology and microstructure of LiMn2O4 particles with a scanning electron microscope (SEM; Hitachi S-800) and transmission electron microscope (ТЕМ; JEOL, 2000FX). We identified the crystalline phase of LiMn2O4 powders using powder X-ray diffraction (XRD; Macscience MXP-3V). We measured the amount of water and organic species in the as-prepared powders by differential thermal analysis and thermogravimetry (DTA-TG; Shimadzu DTG-60). We measured the specific surface area by the BET method (Shimadzu, Tristar3000). We determined the chemical composition of the as-prepared powders by atomic adsorption analysis (Hitachi, Z-5000).
2.3. Electrochemical measurement of lithium secondary batteries
We carried out heat treatment at 750 °С for 2 h under the air because the water and undecomposed salts were included in as-prepared powders. We prepared cathode materials using 46 wt% LiMn204 powders, 46 wt% acetylene black and 8 wt% fluorine resin. We mixed LiMn2C>4 powders with acetylene black and fluorine resin by using a mortar and pressed on either side of a Ti mesh (3 cm2). We used Metal Li (Syotokinzoku) as an anode and the Celgard (Heist Japan) as a separator. We used LiC104 (1 mol/dm3) in propylene carbonate: 1,2-dimethoxyethane (1:1 in volume) as the electrolyte. We built up a lithium secondary battery in globe box under an argon atmosphere [6]. We measured the change of voltage during charge/discharge with a potentiostat (Hosen, Battery cycler) between 3.5 and 4.5 V.
Notes:
particle morphology – морфология(структура) частиц lithium manganate – литий манганата
ultrasonic spray pyrolysis – |
ультразвуковое распыление пиролиза |
metal nitrate and acetate – |
нитрат металла и ацетата |
respectively – соответственно to attain – достигать
double-distilled water – дважды дистиллированная вода mist of starting solution – дымка исходного раствора electrical furnace – электрическая печь
to decompose – разлагать diffraction – дифракция
thermogravimetry – термогравиметрия undecomposed salts – неразложившиеся соли cathode materials – катодные материалы
83
fluorine resin – смола фтора mortar – ступка
Ti mesh – Ti сетка
globe box – сферическая коробка potentiostat – потенциостат
3. Writing the Materials and Methods assumes the application of three types of Description: physical description (which is concerned with physical structures), function description (which is concerned with the purpose of a device and how its parts work), and process description (which is concerned with processes and procedures).
TASK 4.9. Read and translate the following abstracts. They are all taken from Materials and Methods sections of different Medical and Biological published studies. Try to define the types of description used.
a.The liver is the largest organ in the body. It weighs a little more than three pounds in an adult. It is wedge-shaped and is situated under the diaphragm, mostly on the left side of the body, where it is protected by the lower ribs. Somewhat like an intricate chemical factory, the liver takes the particles of glucose (which come from digested starches and sugars) and changes them into another kind of carbohydrate called glycogen, which it then stores. When the body needs sugar, the liver turns the glycogen into glucose again and sends it to the body tissues through the bloodstream.
b.Carbon, the basic element of organic chemistry, undergoes a natural
cycle in the environment. It exists in the form of carbon dioxide in the atmosphere. From there it is absorbed by plants to build carbohydrates in green leaves. When plants burn, and animals breathe out, carbon dioxide passes back into the air. Also in decaying plant and animal remains, carbohydrates are broken down to release carbon dioxide into the atmosphere.
c. Each component of blood has very specialized and important functions. Red blood cells contain hemoglobin, which is a red, iron-rich protein. Hemoglobin enables red blood cells to carry oxygen from the lungs to all parts of the body. Red blood cells give blood its color. When the blood is rich in oxygen it is red, and when there is little oxygen in the blood, the blood is blue. Because blood traveling from the lungs to the body usually contains lots of oxygen, blood in the arteries is normally red. Much of the oxygen is removed from the small capillaries by the body tissues, so blood in the veins tends to be blue in color.
The white blood cells defend the body against disease. They destroy bacteria and foreign material, they stimulate inflammation and assist in the healing process, and they produce proteins called antibodies that destroy bacteria, viruses, and other diseases. WBCs move in and out of the blood stream, depending upon where they are needed.
Platelets help the blood to clot. They group together to form clumps, plugging any holes that develop in blood vessels. Clumps of platelets form a scaffolding upon which a blood clot may form. Formation of a blood clot is a complicated process called coagulation.
84
Plasma is the watery material that carries all other components of the blood within the blood vessels. If water is lost through dehydration, wounds or burns, then the blood can become thickened, almost like sludge, and circulation will be adversely affected.
Notes:
liver – печень
wedge-shaped – клиновидный ribs – ребра
intricate chemical factory – сложный химический завод digested starches – переваренный крахмал carbohydrate – углевод
body tissues – ткани организма
decaying plant – распадающееся растение blood cells – клетки крови
inflammation – воспаление
healing process – процесс заживления platelets – тромбоциты
to clot – свернуться
plugging any holes – подключить любые отверстия clumps – сгустки
sludge – осадок
TASK 4.10. Read and translate the Materials and Methods section of the article from the International Journal of the Society of Powder Technology. What types of Description can you find in it? Give the examples.
FLUIDIZATION BEHAVIOR OF GLASS BEADS UNDER DIFFERENT
VIBRATION MODULES8
A schematic diagram of the experimental apparatus used is shown in Fig. 1. The unit mainly consists of a fluidization chamber (400 mm high, 300 mm wide and 20 mm thick) with a perforated plate distributor and a couple of vibro-motors equipped on a steel plate, which is attached directly to the fluidized bed to impart sinusoidal vibration to the entire bed. The vibro-motors rotated at the same speed and in the opposite rotational direction in order to generate vibration in only one direction, i.e. the vertical or horizontal direction. Thus, the direction of vibration was chosen by the angle at which the two vibromotors were attached. The vibration frequency and amplitude were controlled by adjusting the inverter and the setting location of weights, respectively, inside the vibro-motors. Air was used as the fluidizing gas, and supplied by the compressor via an air filter and an oil eliminator. A pressure tap was mounted near the distributor so that the pressure drops could be measured. The powder used was glass beads with the average diameter of 198 µm and density of 2520 kg/m3. The static bed height was 205 mm in all experiments. The ranges of the operating conditions used in the experiments were determined according to the ranges of parameters normally encountered in the particle coating of vibro-fluidized bed [5].
85

Vertical vibration |
|
Horizontal vibration |
Figure 1. Schematic diagram of experimental apparatus:
(1)compressor, (2) air bed, (3) oil eliminator, (4) air dryer, (5) valve, (6) rotameter,
(7)fluidized bed and (8) vibro-motor
Notes:
fluidization behavior – поведение псевдоожижения glass beads – стеклянные шарики
fluidization chamber – камера флюидизации
perforated plate distributor – перфорированная пластина распределителя to impart – придавать
to attach – прилагать
by adjusting the inverter – путем регулирования инвертора oil eliminator – маслоотделитель
pressure tap – отбор давления, штуцер для измерения давления pressure drops – перепады давлений
vibro-fluidized bed – вибро-кипящий слой
TASK 11. Read and translate the Materials and Methods section of a journal article describing an empirical study relating to Powder Technology research. Then do the following:
1.Make a note in the margin of what is discussed in each paragraph.
2.Underline, preferably in different colors, those sentences and phrases which describe:
∙Overview of the Experiment
∙Population/Sample
∙Location
∙Restrictions/Limiting Conditions
86
∙Sampling Technique
∙Procedures
∙Materials
∙Variables
∙Statistical Treatment.
3.Identify the types of described materials.
4.Analyze the procedures followed in this section:
a.is it clearly described?
b.could you replicate this study based on the researcher's description of the procedure followed? If not, why not?
5.Underline in pencil all the verbs used (except for infinitives). Decide whether they are (1) in the active or passive voice; (2) in the present or past tense.
DYNAMIC RESPONSE OF WELL – MIXED BINARY PARTICULATE SYSTEMS SUBJECTED TO LOW MAGNITUDE VIBRATION9
2. 1. Experimental apparatus and procedure
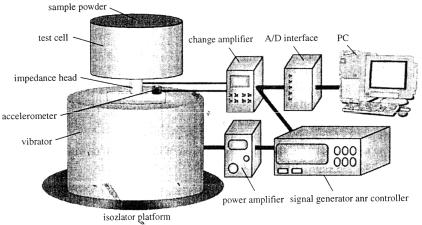
The experimental system is structurally similar to the Top-Cap Method [11 – 13, 22, 30. 31] and the Open-Top-Single-Frequency Method [10, 29]. However, the principle is different. This system is able to perform without the top-cap mass, which enables loosely packed mixture beds to be measured rapidly and conveniently. Figure 1 illustrates the experimental system. A perspex cylindrical test cell was mounted on an impedance head (9311IB; Kistler), consisting of a force transducer, driven by an electromechanical vibrator (V403; Ling Dynamic Systems), with a vertical line of action. The test cell size ranged from 0.01 to 0.08 m for the vessel height and 0.0345 to 0.149 m for the vessel inner diameter to investigate the effect of the vessel shape.
Figure 1. Schematic diagram of the experimental system.
87
The vibration exciter was placed on an isolator platform to prevent the measuring signals from other sources of noise from the general surroundings. Preliminary tests demonstrated that the background noise was negligible within the range of this study. The vibrator was a system with a programmable, feedback controller (DSC4; CED). Feedback control was achieved via the accelerometer (8636C50; Kistler) on the base plate, which provided input signals for the controller to modify and provide output feed to the power amplifier (PA100E; Ling Dynamic Systems) which drove the vibrator, to give a required acceleration at a chosen frequency range. Accelerometer and force transducer were of the piezoelectric type. They were connected via a coupler to an A/D interface (1401; CED), which recorded data at a rate of 12.5 kHz per channel, onto the hard disk of a PC. The sampling frequency was larger than the minimum sampling frequency, i.e. Nyquist frequency, which was 4 kHz within the range of this study, based upon Shannon's sampling theorem [33]. The piezoelectric devices are characterized by very rapid response times with high accuracy and reproducibility, providing the reliable data acquisition.
The sample powders were placed carefully into the test cell by only gravity using a spatula and subsequently levelling the bed surface using a straight edge. Then, a sweep vibration that ranged from 10 to 2000 Hz was applied to the test cell through the base at a chosen sweep of 300 Hz/min with a constant peak acceleration of 0.02 x g [32]. Within this frequency range, the vibrator, force transducer and accelerometer were precise and independent of their structural resonance. During the sweep, data from two measurement devices were monitored as time series on the hard disk of the computer. The number of collected data points for each channel was 5.0 x 106. Subsequently, these time series data were analyzed by fast Fourier transform with a sampling number of 4096 points to give the frequency domain data, from which an apparent mass, defined as a ratio of the base force and base acceleration, was measured on the computer. To ensure the reproducibility of tests, each test was repeated 3 times. The variation of the bulk density for each test was within ± 10% over the range of this study and this range of bulk density is generally referred to as a loose bulk density in the literature [1].
2.2. Sample binary mixtures
Experiments were performed on a range of binary mixtures comprised of low-density polyethylene powder, glass spheres, building sands and rubber powder with the size distributions shown in Fig. 2. The physical properties of the original sample powders are given in Table 1. The polyethylene powder, glass sphere and sands were sieved to provide materials with required size ranges. Table 2 shows the material combinations of binary mixtures used in the tests. A planetary mixer (A901; Kenwood Manufacturing) was used to produce the well-mixed samples. The mixing was performed for 30 min. The mixing homogeneity of samples was confirmed by conventional sampling test. Since the acceleration level of vibration (0.02 x g) employed in this study was low, the structure of particulate beds was unchanged, i.e. the mixing homogeneity was maintained constant during vibration.
88
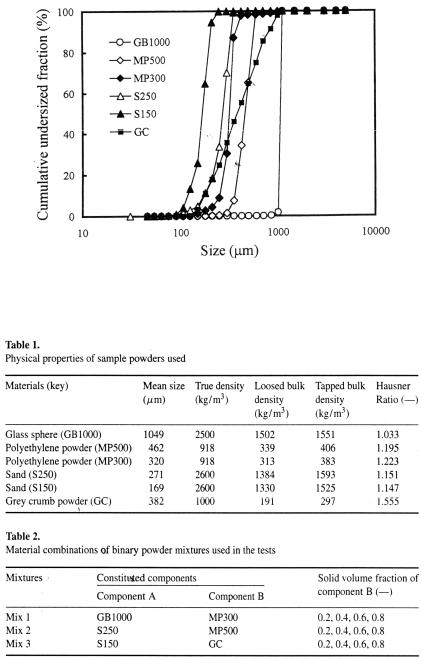
Figure 2. Size distributions of the sample powders used.
89
Notes:
сonveniently – удобно
perspex cylindrical test cell – цилиндрическая испытательная камера из плексигласа/люцита (органическое стекло)
impedance – сопротивление transducer – преобразователь vibration exciter – вибровозбудитель negligible – незначительный
feedback controller – обратная связь с контроллером accelerometer – акселерометр
power amplifier – усилитель мощности piezoelectric type – пьезоэлектрический тип interface – интерфейс, граница раздела фаз bed – слой, пласт, платформа, основание
Nyquist frequency – частота Найквиста
acquisition – сбор, получение, поглощение, слияние gravity – вес, притяжение
spatula – шпатель
sweep vibration – вибрация развертки
frequency domain data – данные частотной области
bulk density – объемная плотность, объемная масса, насыпная масса binary mixtures – бинарные смеси
building sands – строительные пески to sieve – просеивать, сортировать
PRACTICE WRITING YOUR PAPER
TASK 4.12. In previous Units you have begun writing up an original research paper. You have already written the Abstract and the Introduction to your own experimental research paper. Now, carry out your study. Plan and follow a series of procedural steps as determined by your purpose and your research design. Develop and use any instruments (such as surveys, questionnaires, tests, and so on) you need in order to collect data. Finally, when you have completed all the steps and collected all your data, write a procedural description of the methodology you used. Before you write, remember:
1.Procedural descriptions are arranged chronologically.
2.The past tense is usually used to indicate the procedures which were used in the study.
3.The passive voice is commonly used in this section of the research article.
90
Points to consider when writing the Materials and Methods:
∙Don’t mix results with procedures.
∙Omit all explanatory information and background – s ave it for the Discussion.
∙Don’t include information that is irrelevant to the reader (e.g. what colour ice bucket you used).
VOCABULARY AND GRAMMAR AID
PHRASES USED IN WRITING DESCRIPTIONS:
To make strong descriptions we should outline position, weight, structure, colour, composition, size, shape and, functions of an object.
|
|
Position |
|
|
opposite |
|
|
in the middle of |
|
|
on the right of |
|
|
on the left of |
A |
is |
near |
|
|
close to |
|
|
behind |
|
|
in front of |
|
|
under |
|
|
Structure |
|
|
screwed |
|
|
fixed |
|
|
fastened |
X |
is |
linked |
|
|
tied |
|
|
connected |
|
|
attached |
X |
|
consists of |
|
|
contains |
|
|
includes |
|
|
held in place |
X |
|
secured |
|
supported |
|
|
|
|
|
|
suspended |
B
to Y by Z
Y and Z
Y and Z
by Y
91
|
|
joined |
|
X |
|
mounted |
|
|
|
placed |
|
|
|
Colour |
|
|
|
dark |
green. |
|
|
light |
blue. |
X |
is |
pale |
red. |
|
|
bright |
yellow. |
|
|
dull |
|
|
|
Composition |
|
|
metal. |
|
|
steel |
|
|
aluminum. |
|
|
silk |
X |
is made |
of wood |
|
|
plastic |
|
|
glass |
|
|
Size and weight |
|
|
long |
|
|
high |
X |
is 6 cm |
wide |
|
|
length |
|
The |
height width |
|
diameter |
|
|
|
|
|
The |
weight |
|
|
Shape |
|
|
round |
|
|
rectangular |
|
|
triangular |
|
|
semi-circular |
X |
is |
conical |
|
|
spherical |
|
|
hexagonal |
|
|
octagonal |
|
|
oval |
to Y
on Y
on Y
of |
X |
is |
6 cm |
of |
X |
is |
6 Kg |
in shape
92
X is
X is
The
function purpose
The
objective
aim The thermometer
tripod
square
shaped like a
circle triangle semi-circle
Properties
Elastic malleable flexible soluble
a good conductor of electricity/heat corrosion resistant
|
Function |
|
|
||
of |
the thermometer |
is |
to measure the |
||
temperature |
|||||
|
|
|
|
||
of |
the |
tripod |
is to hold the beaker |
||
is |
used |
for |
measuring the temperature |
||
|
|
|
holding the beaker. |
||
SELF-CHECK QUESTIONS
1.What is the purpose of writing the Materials and Methods section?
2.What typical elements of information can be included in the Materials and Methods section?
3.What elements of information are always included in the Materials and Methods section?
4.What differentiates the Materials and Methods section from Results?
5.What do we mean by materials?
6.What is the best way for describing a procedure?
7.What tense and voice forms of verbs are appropriate in this section?
8.Why should the authors avoid using the first person ("I" or "we")?
9.What types of description are used in this section?
10.Materials and Methods section is considered the easiest section to write. Can you agree with this statement?
93
