
Solid Support Oligosaccharide Synthesis
.pdf
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.11 |
|
CONCLUSIONS |
37 |
||||||
|
|
|
O |
OSi(iPr)2- |
|
|
|
|
1) Ac O, coll, THF |
|
|
|
|
|
O |
O |
OSi(iPr)2- |
OBn |
|
||||||||||||
O |
|
|
|
OBn |
|
2 |
|
|
|
|
|
|
|
O |
|
||||||||||||||||
|
|
O |
|
|
|
|
|
2)I(coll)2ClO4, AnthSO2NH2, THF |
|
|
|
|
|
O |
|
||||||||||||||||
|
|
|
|
|
O |
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
O |
NH2 |
|||||
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
3)Bu4NN3, THF |
|
|
|
|
|
|
|
|
|
BnO |
|
|||||||||||
|
|
|
|
HO |
BnO |
|
|
|
|
|
|
|
|
|
|
|
|
AcO |
NHAc |
||||||||||||
|
|
|
|
|
|
|
|
|
|
4) Ac2O, DMAP, THF |
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
31 |
|
|
|
|
|
|
5)HS-(CH2)3-SH, Hunig's, DMF |
|
|
|
|
88 |
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
O OR |
|
|
CbzAlaLeu |
AsnLeuThr(OBn)OAll |
|
|
|
|
|
|
|
|||||||||||
|
|
75, IIDQ |
|
O |
|
|
|
OBn |
|
|
|
|
|
|
|
||||||||||||||||
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
O NH |
|
|
|
|
|
|
|
|
|
HF-Py |
|
|
|||||
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
89a R = SiPh2- |
|
|
|
|
|
|
|||||||||||
|
|
CH2Cl2 |
|
|
|
AcO |
BnO |
|
|
|
|
|
|
anisole |
|
|
|||||||||||||||
|
|
|
|
|
|
NHAc 89b R = H |
|
|
|
|
|
|
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH2Cl2 |
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
OTIPS |
|
|
|
|
|
|
|
|
OTIPS |
|
|||||||||||
|
|
|
O |
OR |
|
|
|
|
|
|
|
1) - 5) as above |
|
|
|
|
O |
OR |
|
||||||||||||
O |
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
||||||||||||||||
|
|
|
|
O |
|
|
|
|
|
|
|
|
O AcO |
O |
NH |
||||||||||||||||
|
O |
HO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
O |
2 |
|||||||
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
HO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
AcO |
NHAc |
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
56: R = Si(iPr)2- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
91: R = Si(iPr)2- |
|
||||||||||
|
|
|
90: R = Si(iPr) |
O-CH - |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
92: R = Si(iPr)2O-CH2- |
||||||||||||
|
|
|
|
|
|
2 |
|
|
2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CbzAlaLeu |
AsnLeuThr(OBn)OAll |
|
|
|
|
|
|
|
||||||||
|
|
|
75, IIDQ |
|
|
|
|
|
|
|
|
|
OTIPS |
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
O |
O |
OR |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
O AcO |
O |
NH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
CH2Cl2 |
|
|
|
|
|
|
91: R = Si(iPr)2- |
|
|
|
|
|
HF-Py |
|
||||||||||||||
|
|
|
|
|
|
|
O |
|
|
|
O |
NHAc |
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
AcO |
|
92: R = Si(iPr)2O-CH2- |
|
|
anisole |
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
93: R = H |
|
|
|
|
|
|
|
|
|
CH2Cl2 |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Scheme 2.23 Solid-phase synthesis of glycopeptides.
give glycopeptide 86. Retrieval from the solid support afforded trisaccharide– octapeptide 87 in 18% overall yield from 10.
The methodology described above has proved to be effective for other glycosylation sequences. Scheme 2.23 shows conversion of a variety of disaccharides to their respective glycopeptides. For example, lactal derivative 31 was transformed into a lactosamine-linked glycopeptide where C6 of the glucosamine residue is protected as its benzyl ether, in 24% overall yield. Disaccharides 56 and 90, with a β-(1→3) glycosylation pattern, differ in their mode of attachment to the solid support. In compound 56 the linkage was established via a silyl ether, while in 90 it was fashioned via a silylene linkage. They can both be elaborated to disaccharide–pentapeptide 93 in 20% and 44% yields, respectively, based on the initial loading of polymer-bound galactal carbonate.
2.11 CONCLUSIONS
In this chapter we have described the progress that our laboratory has made in the solid support synthesis of oligosaccharides and glycopeptides using glycal assembly. Protocols for the effective transformation of glycals into powerful glycosyl donors such as 1,2-anhydrosugars and thioethylglycosides have been developed. A variety of
38 THE GLYCAL ASSEMBLY METHOD ON SOLID SUPPORTS
glycosidic linkages may now be fashioned in a selective manner and bring complex structures within reach. The flexibility and capabilities of our synthetic approach were demonstrated on some select structures of biological interest.
Glycal assembly on a solid support eliminates the repetitive purifications usually associated with oligosaccharide synthesis. As a method, it has a certain generality as it does not require any specific enzymes or complex starting materials. Both natural and nonnatural sugars may be used in the constructions.
A number of challenges still remain before a flexible, high-yielding, and absolutely selective strategy for the synthesis of oligosaccharides on the solid support becomes available. Once these problems are solved, the construction of an automated oligosaccharide synthesizer will become feasible.
The rapid access to complex glycoconjugates will open the door to detailed studies concerning the structure and function of this class of biooligomers.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (grant numbers AI 16943, CA 28824, HL 25848). We thank our coworkers whose names are cited in the footnotes.
REFERENCES
1.(a) Glycoconjugates: Composition, Structure, and Function, Allen, H. J., and Kisailus,
E.C. (Eds.), Marcel Dekker, New York, 1992; (b) Neoglycoconjugates: Preparation and Applications, Lee, Y. C., and Lee, R. T., (Eds.), Academic Press, London, 1994; (b) Kobata, A., Acct. Chem. Res. 26, 319–324 (1993).
2.Varki, A., Glycobiology 3, 97–130 (1993).
3.(a) Phillips, M. L., Nudelman, E., Gaeta, F. C. A., Perez, M., Singhal, A. K., Hakomori, S., and Paulson, J. C., Science 250, 1130–1132 (1990); (b) Borman, S., Chem. Eng. News 27 (June 28, 1993); (c) Lasky, L. A., Science 258, 964–969 (1992); (d) Miller, D. J., Macek, M. B., and Schur, B. D., Nature 357, 589–593 (1992); (e) Schulze, I. T., and Manger, I. D., Glycoconjugate J. 9, 63–68 (1992); (f) Taki, T., Hirabayashi, Y., Ishikawa, H., Kon, S., Tanaka, Y., and Matsumoto, M., J. Biol. Chem. 261, 3075–3078 (1986); (g) Spohr, U., and Lemieux, R. U., Carbohydr. Res. 174, 211–223 (1988); (h) Levy, D. E., Tang, P. C., and Musser, J. H., in Ann. Rep. Med. Chem., Hagmann W. K. (Ed)., Academic Press, San Diego, 1994, Vol. 29, pp. 215–224.
4.Opdenakker, G., Rudd, P. M., Ponting, C. P., and Dwek, R. A.,FASEB J. 7, 1330–13370 (1993).
5.For a review on peptides see: Atherton, E., and Sheppard, R. C., Solid Phase Peptide Synthesis: A Practical Approach; IRL Press, Oxford Univ. Press, Oxford, England, 1989, p. 203; for nucleotides, see Caruthers, M. H., Science 230, 281–285 (1985).
6.The following is a list of reviews: (a) Boons, G.-J., Tetrahedron 52, 1095–1121 (1996);
(b) Kunz, H., and von dem Bruch, K., Meth. Enzymol. 2473–2530 (1994); (c) St. Hilaire,
P.M., and Meldal, M., Angew. Chem., Int. Ed. 39, 1163–1179 (2000); (d) Paulsen, H.,
Angew. Chem., Int. Ed. 29, 823–842 (1990); (e) Wong, C.-H., Pure Appl. Chem. 67,
REFERENCES 39
1609–1616 (1995); (f) Meldal, M., and Bock, K., Glycoconjugate 11, 59–63 (1994); (g) Kappes, T., and Waldmann, H., Top. Curr. Chem. 186, 65–86 (1996); (h) Danishefsky,
S.J., and Roberge, J. Y., in Glycopeptides and Related Compounds: Synthesis, Analysis and Applications, Large, D. G., and Warren, C. D. (Eds.), Marcel Dekker; New York, 1997, p. 245 and references therein; (i) Seeberger, P. H., and Danishefsky, S. J., Acc. Chem. Res. 31, 685–695 (1998).
7.Halcomb, R. L., and Danishefsky, S. J., J. Am. Chem. Soc. 111, 6661–6666 (1989).
8.Chan, T.-H., and Huang, W.-Q., J. Chem. Soc. Chem. Commun. 909–911 (1985).
9.Savin, K. A., Woo, J. C. G., and Danishefsky, S. J.,J. Org. Chem. 64, 4183–4186 (1999).
10.Merrifield, R. B., J. Am. Chem. Soc. 85, 2149–2154 (1963).
11.(a) Wang, S. S., J. Am. Chem. Soc. 95, 1328–1333 (1973); (b) Lu, G., Mojsov, S., Tam,
J.P., and Merrifield, R. B., J. Org. Chem. 46, 3433–3436 (1981).
12.Danishefsky, S. J., McClure, K. F., Randolph, J. T., and Ruggeri, R. B., Science 260, 1307–1309 (1993).
13.Murray, R. W., and Jeyaraman, R., J. Org. Chem. 50, 2847–2853 (1985).
14.Seeberger, P. H., Beebe, X., Sukenick, G., Pochapsky, S., and Danishefsky, S. J.,Angew. Chem. Int. Ed., 36, 491–493 (1997).
15.Race, R. R., and Sanger, R., Blood Groups in Man, 6th ed., Blackwell, Oxford, 1975.
16.(a) Phillips, M. L., Nudelman, E., Gaeta, F. C. A., Perez, M., Singhal, A. K., Hakomori, S., and Paulson, J. C., Science 250, 1130–1132 (1990); (b) Hirabayashi, Y., Hyogo, A., Nakao, T., Tsuchiya, K., Suzuki, Y., Matsumoto, M., Kon, K., and Ando, S., J. Biol. Chem. 265, 8144–8151 (1990); (c) Spohr, U., and Lemieux, R. U., J. Biol. Chem. 174, 211–237 (1988); (d) Polley, M. J., Phillips, M. L., Wagner, E., Nudelman, E., Singhal,
A.K., Hakomori, S., and Paulson, J. C., Proc. Natl. Acad. Sci. (USA) 88, 6224–6228 (1991); (e) Hindsgaul, O., Norberg, T., Le Pendu, J., and Lemieux, R. U., Carbohydr. Res. 109, 109–142 (1982).
17.For a review, see Lloyd, K. O., Am. J. Clin. Pathol. 87, 129–139 (1987).
18.For syntheses of H-type determinants, see (a) Petrakova, E., Spohr, U., and Lemieux, R. U., Can. J. Chem. 70, 233–240 (1992); (b) Zemlyanukhina, T. V., and Bovin, N. V., Bioorg. Khim. 16, 1096–1104 (1990).
19.Randolph, J. T., and Danishefsky, S. J., Angew. Chem., Int. Ed. 33, 1470–1473 (1994).
20.Seeberger, P. H., Eckhardt, M., Gutteridge, C. E., and Danishefsky, S. J., J. Am. Chem. Soc. 119, 10064–10072 (1997). For the activation of thiodonors, see (a) Lonn, H., Carbohydr. Res. 139, 115–121 (1985); (c) Lonn, H., J. Carbohydr. Chem. 6, 301–306 (1987); (d) Fugedi, P., and Garegg, P. J., Carbohydr. Res. 149, C9–C12 (1986).
21.Kunz, H., and Harreus, A., Liebigs Ann. Chem. 41–48 (1982).
22.Kunz, H., and Harreus, A., Liebigs Ann. Chem. 717–730 (1986).
23.Zheng, C., Seeberger, P. H., and Danishefsky, S. J., J. Org. Chem. 63, 1126–1130 (1998).
24.(a) Griffith, D. A., and Danishefsky, S. J., J. Am. Chem. Soc. 112, 5811–5819 (1990);
(b) Hamada, T., Nishida, A. and Yonemitsu, O.,J. Am. Chem. Soc. 108, 140–145 (1986).
25.Zheng, C., Seeberger, P. H., and Danishefsky, S. J., Angew. Chem., Int. Ed. 37, 786–789 (1998).
26.Boren, T., Falk, P., Roth, K. A., Larson, G., and Normark, S., Science 262, 1892–1895 (1993).
40THE GLYCAL ASSEMBLY METHOD ON SOLID SUPPORTS
27.Alper, J., Science 260, 159–160 (1993).
28.(a) Graham, D. Y., Lew, G. M., Klein, P. D., Evans, D. G., Evans, D. J., Saeed, Z. A., and Malaty, H. M., Ann. Int. Med. 116, 705–708 (1992); (b) Hentschel, E., Brandstatter, G., Dragosics, B., Hirschl, A. M., Nemeg, H., Schutze, K., Taufer, M., and Wurzer, H.,
N.Engl. J. Med. 328, 308–312 (1993).
29.Sharon, N., in The Lectins: Properties, Functions and Applications in Biology and Medicine, Liener, I. E., Sharon, N., and Goldstein, I. J. (Eds.), Academic Press, New York, 1986, pp. 494–525.
30.Bernstein, M. A., and Hall, L. D., Carbohydr. Res. 78, C1–C4 (1980).
31.Bremer, E. G., Levery, S. B., Sonnino, S., Ghidoni, R., Canevari, S., Kannagi, R., and Hakomori, S., J. Biol. Chem. 259, 14773–14777 (1984).
32.Menard, S., Tagliabue, E., Canevari, S., Fossati, G., and Colnaghi, M. I., Cancer Res. 43, 1295–1300 (1983).
33.Kudryashov, V., Ragupathi, G., Kim, I. J., Breimer, M. E., Danishefsky, S. J., Livingston, P. O., and Lloyd, K. O., Glycoconj. J. 15, 243–249 (1998).
34.Zhang, S., Cordon-Cardo, C., Zhang, H. S., Reuter, V. E., Adluri, S., Hamilton, W. B., Lloyd, K. O., and Livingston, P. O., Int. J. Cancer 73, 42–49 (1997).
35.Park, T. K., Kim, I. J., Hu, S., Bilodeau, M. T., Randolph, J. T., Kwon, O., and Danishefsky, S. J., J. Am. Chem. Soc. 118, 11488–11500 (1996).
36.Ragupathi, G., Park, T. K., Zhang, S., Kim, I. J., Graber, L., Adluri, S., Lloyd, K. O., Danishefsky, S. J., and Livingston, P. O., Angew. Chem., Int. Ed. 36, 125–128 (1997).
37.Ragupathi, G., Slovin, S. F., Adluri, S., Sames, D., Kim, I. J., Kim, H. M., Spassova, M., Bornmann, W. G., Lloyd, K. O., Scher, H. I., Livingston, P. O., and Danishefsky, S. J.,
Angew. Chem., Int. Ed. 38, 563–566 (1999).
38.Williams W., Zhang S., and Danishefsky, S. J., unpublished results.
39.Kunz, H., Angew. Chem., Int. Ed. 26, 294–308 (1987).
40.Ioffe, E., and Stanley, P., Proc. Natl. Acad. Sci. (USA) 93, 11041–11046 (1996).
41.Imperiali, B., and Rickert, K. W., Proc. Natl. Acad. Sci. (USA) 92, 97–101 (1995).
42.Meldal, M., Curr. Opin. Struct. Biol. 4, 710–718 (1994).
43.For selected examples, see (a) Cohen-Anisfeld, S. T., and Landsbury, P. T.,J. Am. Chem. Soc. 115, 10531–10537 (1993); (b) Vetter, D., Tumelty, D., Singh, S. K., and Gallop,
M.A., Angew. Chem., Int. Ed. 34, 60–63 (1995).
44.Lloyd-Williams, P., Gairi, M., Albericio, F., and Giralt, E., Tetrahedron 49, 10069–10078 (1993).
45.(a) Roberge, J. Y., Beebe, X., and Danishefsky, S. J., Science 269, 202–204 (1995); (b) Roberge, J. Y., Beebe, X., and Danishefsky, S. J., J. Am. Chem. Soc. 120, 3915–3927 (1998).
Solid Support Oligosaccharide Synthesis and Combinatorial Carbohydrate Libraries. Edited by Peter H. Seeberger Copyright © 2001 John Wiley & Sons, Inc.
ISBNs: 0-471-37828-3 (Hardback); 0-471-22044-2 (Electronic)
3The Sulfoxide Glycosylation Method and its Application to Solid-Phase Oligosaccharide Synthesis and the Generation of Combinatorial Libraries
CAROL M. TAYLOR
Institute of Fundamental Sciences, Massey University, Palmerston North,
New Zealand
3.1 INTRODUCTION
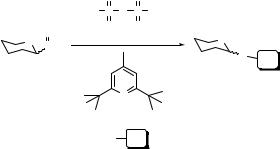
The sulfoxide method was introduced by Kahne and coworkers,1 and was heralded as “a new method for rapid glycosylation of unreactive substrates in high yield under mild conditions.” The reaction involves the sulfoxide donor [sulfoxide (I)], an activating agent (usually triflic anhydride), a hindered, nonnucleophilic base (2,6-di-tert-butyl-4-methylpyridine, DTBMP) and a nucleophilic acceptor (most often an alcohol) (Scheme 3.1). The glycosylation of sterically hindered steroidal alcohols, phenols and the N-glycosylation of an acetamide was reported (Table 3.1).
|
|
O |
O |
|
|
|
|
F3C S O S CF3 |
|
|
|
|
O |
O |
O |
|
|
O |
(Tf2O) |
O |
|
||
SPh |
|
||||
|
|
|
O |
|
|
glycosyl |
|
|
R |
||
|
|
|
|||
|
|
|
|
||
sulfoxide (I) |
|
|
O-glycoside (II) |
||
N
(DTBMP)
HO R
Scheme 3.1 The sulfoxide glycosylation method.
41
42 THE SULFOXIDE GLYCOSYLATION METHOD
TABLE 3.1 Glycosylation of Unreactive Substrates
|
|
Yield and Ratio |
Glycosyl Acceptor |
Glycosyl Donor Reaction Solvent |
of Anomers |
|
|
|
|
|
OBn |
O |
|
|
|
Me |
H |
BnO |
O |
toluene |
|
|
|
|
SPh |
||||
Me |
H |
Me |
COOMe |
BnO |
|
CH2Cl2 |
|
|
OBn |
||||||
|
|
|
|
||||
H |
|
H |
|
Sulfoxide 2 |
|
CH3CN |
|
H OH |
|
|
OPiv |
|
|
||
1 |
|
|
O |
|
|||
|
|
|
PivO |
O |
|
||
EtOOCO |
|
|
|
SPh |
|
||
|
|
|
PivO |
|
CH2Cl2 |
||
|
|
|
|
OPiv |
|||
|
|
|
|
|
|||
Sulfoxide 3
86%; 27:1 (α:β) 80%; 1:3 (α:β) 50%; 1:8 (α:β)
83%; all β
|
|
Me |
H |
|
|
|
|
|
|
|
|
Me |
H |
Me |
COOMe Sulfoxide 2 |
|
58%; α, α |
H |
|
|
toluene |
||
HO |
H |
|
|
|
|
H |
|
|
|
||
OH |
|
|
|
||
|
4 |
|
|
|
|
|
|
|
|
|
EtOOCO
O O
5
OH
Sulfoxide 2 |
toluene |
78%; 9:1 (α:β) |
Sulfoxide 3 |
CH2Cl2 |
60%; all β |
|
|
|
|
|
toluene |
70%; 2:1 (α:β) |
|
|
|
|
Sulfoxide 2 |
|
|
H3C |
|
|
CH3 |
|
|
|
|
|
OH |
Sulfoxide 3 |
CH2Cl2 |
80%; all β |
|
|
6 |
|||||
|
|
|
|
|
|
|
|
O |
|
|
|
||
CH3 |
NHSi(CH3)3 |
Sulfoxide 2 |
toluene |
66%; 5:1 (α:β) |
||
|
|
|
|
|
||
|
|
7 |
|
|
|
|
|
|
|
|
|
|
|
Since 1989, the sulfoxide method has been investigated extensively by the laboratories of Kahne and Crich and employed by many others.
Figure 3.1 depicts some oligosaccharides that were synthesized using the sulfoxide glycosylation method. Chemical yields and stereoselectivity, for glycoside formation, are highlighted. Crich and Dai reported the synthesis of a trisaccharide 8, a component of the Hyriopsis schlegelli glycosphingolipid; one of the linkages was successfully established using the sulfoxide method.2 Yan and Kahne synthesized trisaccharide 9 en route to Lewisx.3 Zhang and coworkers applied the sulfoxide glycosylation method to the assembly of compound 10, the nephritogenoside trisaccharide unit.4 Berkowitz et al. considered the sulfoxide method in the assembly of artificial disaccharides such as 11.5 Kim et al. constructed the three glycosidic linkages in the calicheamicin oligosaccharide 12.6
3.1 INTRODUCTION 43
OBz |
OBz |
|
|
AcO |
OAc |
|
65% β |
|
|
|
|
|
|
|
|
OAc |
|||
|
O |
|
87% β |
|
|
O |
|
||
|
|
|
AcO |
OO |
O |
||||
OBz |
7% α |
|
|
|
|||||
|
|
O |
OBn |
|
OAc |
N |
|
||
Ph O |
|
O |
|
|
O |
3 |
SPh |
||
O |
|
|
O |
O |
|
|
|
OBn |
|
BnO |
|
|
|
|
OBn |
|
85% α |
||
|
|
BnO |
|
|
|
||||
|
|
|
|
BnO |
BnO |
|
|
|
|
|
|
8 |
|
|
|
|
9 |
|
|
|
|
|
OMe |
|
|
|
|
||
OBn |
68% α |
|
|
|
|
98% |
|||
|
O |
|
|
|
|
|
|||
BnO |
|
17% β |
|
|
|
|
O |
all β |
|
BnO |
|
|
|
|
BnO |
|
|
||
BnO |
|
|
|
BnO |
|
O |
|||
|
O |
|
|
|
|||||
|
|
78% |
PivO |
|
|||||
PivO |
|
|
O |
|
|
|
O |
|
|
PivO |
|
O |
all β |
|
|
|
|
O |
|
|
|
PivO |
|
|
|
|
O |
|
|
|
|
|
BnO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OO |
|
|
|
|
BnO |
BnO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10 |
|
N3 |
|
|
|
11 |
|
|
|
99% |
|
O |
|
|
|
|
|
|
|
I |
S |
O |
H |
|
|
|
|
|
|
all β |
|
O |
|
||||
|
|
|
N |
|
|
||||
|
|
|
|
O |
|
|
|||
|
|
|
O |
OMeOH |
HO |
|
OMe |
||
|
|
|
|
O |
|
||||
|
|
|
|
|
65% α |
||||
|
|
O |
OMe |
|
|
|
O |
||
HO |
39% β EtNH |
|
5% β |
||||||
|
|
||||||||
|
|
|
|
||||||
|
|
OMe OH |
|
3% α |
MeO |
|
|
||
|
|
|
|
|
12 |
|
|
|
|
Figure 3.1 Oligosaccharides synthesized using the sulfoxide glycosylation method.
|
|
|
|
|
|
|
|
O |
|
|
|
|
O |
|
|
|
HN |
|
|
O |
|
|
|
|
|
O |
N |
|
|
BzO O SPh |
|
HN |
|
TMSOTf |
|
|
|||
|
|
ClCH2CH2Cl |
|
BzO O |
* = 13C |
||||
* |
|
|
|
|
|
|
* |
||
+ |
O |
N |
|
RT, 5 mins |
|
|
|||
|
|
|
|
|
|
|
|||
BzO OBz |
|
|
SI(CH3)3 |
(92%) |
|
BzO |
OBz |
|
|
13 |
|
|
14 |
|
|
15 |
|
||
O |
|
|
NHAc |
|
TMSOTf |
|
|
O |
N NHAc |
|
|
|
|
|
|
||||
S |
|
+ |
N |
|
ClCH2CH2Cl |
|
|
S |
N |
TBDPSO |
|
|
|
0 ºC |
|
TBDPSO |
|
|
|
|
O |
N |
|
|
|
|
|||
|
|
|
|
|
|
|
|
||
HO |
|
|
Si(CH3)3 |
(74%) |
|
|
HO |
|
|
16 |
|
|
17 |
|
|
|
|
18 |
|
|
|
|
|
|
|
|
|
(2.6:1.0, α:β) |
|
Scheme 3.2 Synthesis of nucleoside analogs.
Sulfoxides have also been used in the synthesis of nucleoside analogs (Scheme 3.2). Chanteloup and Beau reported the synthesis of ribofuranosyl sulfoxide 13 and its use in the glycosylation of a series of silylated pyrimidine and purine bases.7 Although 16 is not an anomeric sulfoxide, its reaction with cytosine derivative 17 is conceptually related.8
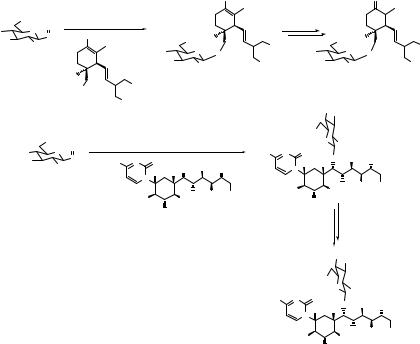
44 |
THE SULFOXIDE GLYCOSYLATION METHOD |
|
|
|
|
|
|
|
|||||||
|
|
|
|
1. 1.8 eq. Tf2O |
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
5.9 eq. DTBMP |
|
OTIPS |
|
|
|
|
|
O |
|
|
|
|
|
|
|
4Å molecular sieves |
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
OPMB |
|
|
CH2Cl2, -90 °C |
|
|
|
|
|
|
|
|
|
||
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
||
PMBO |
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SPh |
2. |
OTIPS |
|
|
|
|
|
|
|
|
|
|||
PMBO |
|
|
OPMB |
|
|
|
|
OH |
|
|
|
||||
|
OPMB |
|
|
|
|
OTIPS |
|
|
|
OH |
|||||
|
|
|
|
PMBO |
O |
|
HO |
O |
|
||||||
|
19 |
|
|
|
O |
O |
|||||||||
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
PMBO |
OPMB |
OTIPS |
HO |
|
OH |
|
OH |
||
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
21 |
|
|
|
|
(-)-cassioside (22) |
||
|
|
|
|
HO |
OTIPS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
20 |
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OTIPS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(50%) |
|
|
|
|
PivO N3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
1. Tf2O, DTBMP |
|
|
|
|
PivO |
|
|
|
|
|
|
|
OPiv |
|
|
|
|
|
|
O |
OPiv |
|
|
||
|
|
|
O |
O |
|
CH2Cl2, -78 °C |
|
|
|
|
|
|
|
||
|
PivO |
|
|
|
|
AcNH |
N |
O |
|
|
|
|
|||
|
|
SPh 2. AcNH N O |
|
|
O |
OAc OAc |
|
||||||||
|
N |
|
|
|
|
|
|
H H |
|
||||||
|
3 |
OPiv |
|
|
OH OAc OAc |
|
N |
|
|
|
|
||||
|
|
|
|
H H |
|
|
|
|
|
|
|||||
|
|
|
23 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
|
|
|
|
|
OBn OAc OAc |
||||
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
OBn OAc OAc |
|
AcO |
|
N3 |
|
|
|
|
|
|
|
|
|
|
AcO |
N3 |
|
|
|
OAc |
|
25 |
|
|
|
|
|
|
|
|
OAc |
24 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(41%) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
HO NH2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
HO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
OH |
|
|
|
|
|
|
|
|
|
|
|
H2N |
N |
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
H O |
OH OH |
|||||
|
|
|
|
|
|
|
|
|
|
|
H |
||||
|
|
|
|
|
|
|
|
|
|
|
N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
HO |
|
OH OH |
OH |
||
|
|
|
|
|
|
|
|
|
|
NH2 |
|
|
|||
OH hikizimycin (26)
Scheme 3.3 Synthesis of glycoside natural products.
The sulfoxide method has come to the rescue in the syntheses of some complex glycoside natural products, where many other glycosylation methods failed. Two examples are shown in Scheme 3.3. Boeckman and Liu reported an enantioselective synthesis of cassioside (22).9 While the focus of the synthesis was the assembly of the aglycone 20, utilizing a highly diastereoselctive cycloaddition of a trienol silyl ether, the glycosylation turned out to be a challenge in its own right. They initially performed this glycosylation using the perbenzylated glucose sulfoxide 2 (see Table 3.1); but found that the hydrogenolytic removal of the benzyl protecting groups was not compatible with other functionality in the molecule. Similar problems were reported in the synthesis of ciclamycin 0.10 The solution, in both cases, was found in the p-methoxybenzyl ether protecting group, which can be removed oxidatively.11 Ikemoto and Schreiber encountered a challenging glycosylation in their synthesis of hikizimycin (26); sterically hindered alcohol 24 was ultimately glycosylated using sulfoxide 23.12
3.2 SYNTHESIS OF SULFOXIDE DONORS
The preparation of thioglycosides was thoroughly reviewed by Garegg.13 These versatile glycosides can be used directly in glycosylation reactions, or can be converted to a number of other glycosyl donors, including sulfoxides.
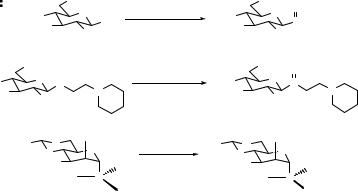
3.3 MECHANISM OF THE SULFOXIDE GLYCOSYLATION 45
AcO
AcO
|
|
OBn |
H2O2, Ac2O |
OBn |
O |
|
BnO |
|
O |
BnO |
O |
||
|
|
|
||||
|
SPh |
|
|
SPh |
||
BnO |
|
CH2Cl2, RT, 4h |
BnO |
|
||
|
OBn |
OBn |
||||
|
|
|
||||
|
|
27 |
(95%) |
|
2 |
|
OAc |
|
|
1 eq. Br2 |
OAc |
O |
|
O |
|
|
|
O |
||
S |
|
AcO |
S |
|||
|
|
|||||
|
|
|
|
|||
OAc |
N |
KHCO3 |
AcO |
|
N |
|
|
OAc |
|||||
28 |
|
|
H2O, CH2Cl2 |
|
|
29 |
|
|
(61%) |
|
|
||
|
|
|
|
|
|
|
Ph O |
OTBS |
|
Ph O |
OTBS |
||
O |
|
O |
||||
O |
|
|
O |
|||
BnO |
Et |
|
BnO |
|
Et |
|
|
|
|
|
|
||
|
|
S |
|
|
|
S |
|
30 |
|
31 |
|
+ |
|
|
|
|
O- |
|||
Scheme 3.4 Synthesis of sulfoxide donors.
Thioglycosides are most commonly oxidized to a mixture of diastereoisomeric sulfoxides using m-CPBA.3 Care must be taken to monitor and minimize overoxidation to the sulfone; low temperatures are usually required. Kakarla et al. have used H2O2/Ac2O/SiO2 in dichloromethane to produce sulfoxide 2 in excellent yield on very large scale.14 Juodvirsis et al. prepared mixtures of sulfoxides (e.g., 29) and sulfones using Br2/KHCO3 (Scheme 3.4).15 Interestingly, Crich and coworkers employed a number of reagents (m-CPBA, monoperoxyphthalate [MMPP], sodium periodate and Oxone®) to oxidize α-mannopyranosyl thioglycosides (e.g., 30), and observed stereoselective formation of the R-sulfoxides.16 This was rationalized on the basis of the exo-anomeric effect, which favors the conformation in which the pro-R lone pair on sulfur is more exposed to attack by oxidizing agents.
3.3 MECHANISM OF THE SULFOXIDE GLYCOSYLATION
A simplistic view of the mechanism is depicted in Scheme 3.5. The sulfoxide (I) is activated by triflic anhydride to generate a sulfonium salt (III). It was observed early on that the outcome of the glycosylation was not influenced by the configuration at the two variable stereogenic centers in the sulfoxide donor (i.e., anomeric carbon and sulfur).1 This was taken as evidence for the intermediacy of the oxycarbenium ion (V), which arises from rapid elimination of an alkylsulfenyl triflate(IV) from III. Reaction of V with a nucleophile then gives rise to a mixture of α- and β-glycosides (II).
The generation of phenylsulfenyl triflate(IV) (R = Ph) can be a problem if there are thioglycosides in the starting materials or product, as these functional groups can be activated as glycosyl donors by phenylsulfenyl triflate. This problem can be dealt with in two ways: inclusion of a scavenger for phenylsulfenyl triflate (e.g., 4-allyl-1,2-dimethoxybenzene, 32) or by ensuring that the thioglycosides are sterically hindered and/or electronically deactivated.10
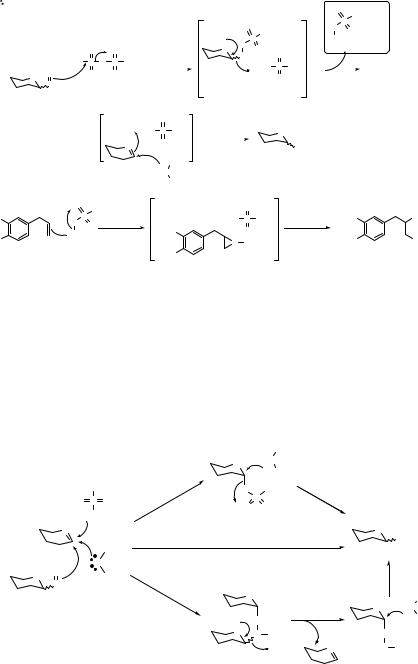
46 THE SULFOXIDE GLYCOSYLATION METHOD
|
|
|
|
|
|
|
|
|
|
|
|
O |
CF3 |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
O |
|
|
O |
S |
|
alkyl |
|
|
|
|
|
|
|
|
|
CF |
|
O |
|
||||
|
|
|
|
|
.. |
|
S |
3 |
|
SR |
sulfenyl |
||||
|
|
O |
O |
|
|
|
O |
O |
O |
|
|
triflate (IV) |
|||
|
|
|
|
|
SR |
|
|
|
|
|
|||||
|
F3C |
S O S CF3 |
|
|
|
|
O |
|
|
|
|
|
|||
|
|
|
|
|
+ |
|
-O S |
CF3 |
|
|
|
|
|||
O |
O |
O |
O |
|
|
|
|
|
|
|
|
|
|
||
|
|
|
sulfonium |
O |
|
|
|
|
|
||||||
SR |
|
triflic |
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
triflate salt (III) |
|
|
|
|
|
|||||
sulfoxide (I) |
anhydride |
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
||
|
|
|
+ |
-O S CF3 |
|
|
O |
|
|
|
|
|
|||
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
O |
|
|
|
|
|
|
|
OR |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
oxycarbenium |
H |
|
|
glycoside (II) |
|
|
|
|||||
|
|
|
O |
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
ion (V) R
O
CF3
MeO O S
O
 SR
SR
MeO
4-allyl-1,2-dimethoxy- benzene (32)
|
O |
aqueous |
|
|
|
-O S CF3 |
MeO |
OH |
|
|
work-up |
|||
MeO |
+ O |
|
MeO |
SR |
|
S R |
|
MeO
episulfonium ion (VI)
Scheme 3.5 Simplistic mechanism for the sulfoxide glycosylation.
In attempts to improve the yields of problematic glycosylation reactions, it has become clear that the mechanism is much more complex than Scheme 3.5 suggests. In essence, the oxycarbenium ion(V) is susceptible to attack by nucleophiles other than the glycosyl acceptor (Scheme 3.6). Two intermediates for which there is experimental evidence are glycosyl triflates(VII)17 and glycosyl sulfenates(IX).18
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
triflate |
|
|
O |
O |
|
|
||
|
|
|
|
|
|
|
R |
|
|
|||
|
|
|
|
anion |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
CF3 |
|
|
O |
CF |
S 2-like |
|
||
|
|
|
O S O |
|
a |
|
S |
3 |
N |
|
||
|
|
|
|
|
O |
O |
|
|
|
|||
|
|
|
|
-O |
|
|
|
|
|
|
||
|
|
+ |
|
|
|
|
glycosyl |
|
|
|
||
|
|
|
a |
|
|
|
|
|
|
|||
oxycarbenium |
|
O |
|
|
|
|
triflate (VII) |
|
O |
|||
|
|
|
|
|
|
|
||||||
ion (V) |
|
|
|
b |
H |
|
b |
|
|
|
|
OR |
|
|
|
|
|
|
|
glycoside (II) |
|||||
|
|
|
|
|
|
|
|
|
|
|||
|
O |
|
c |
O |
|
|
|
|
|
|
|
|
O |
|
|
R |
|
|
|
|
|
|
|
||
SR |
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
O |
|
|
|
|||
sulfoxide |
|
|
|
|
c |
|
|
|
H |
|||
|
|
|
|
|
|
|
|
O |
||||
(I) |
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
.. |
O |
|
|
R |
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
O S R |
|
O |
||
|
|
|
|
|
|
|
|
|
+ |
|
+ |
S R |
|
|
|
|
|
|
|
|
|
|
|
O |
|
sulfenate (IX)
(VIII)
oxycarbenium ion (V)
Scheme 3.6 Triflate and sulfenate intermediates in the sulfoxide glycosylation.
