
Phencyclidine An Update Editor Doris H. Clouet
.pdf
FIGURE 1. Dose-response curve for rates trained to discriminate various doses of PCP from vehicle administered 30 minutes before the test. N=10/dos.
As the dose of PCP was increased, more rats generalized the treatment to that produced by the training dose, so that, when tested with doses comparable to the training condition, essentially all animals completed their first 10 responses on the PCP-appropriate lever. At higher doses of PCP, a significant reduction in the number of responses emitted during the 15-minute session was observed. The rate of responding in animals trained at the lowest dose (1.0 mg/kg) appeared to be more sensitive to disruption by PCP than in those animals trained at higher doses (figure 2). However, no tolerance to this rate-disrupting effect of PCP was seen over the course of the experiments.
138
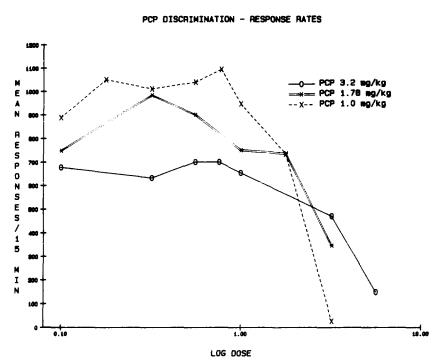
FIGURE 2. Dose-response curve for the effects of PCP on response rates in rats trained to discriminate various doses of PCP from vehicle. N=10/dose.
Generalization tests indicated that a number of compounds were able to substitute for PCP (table 1). Ketamine and tiletamine, which are structurally similar to PCP, produced dose-dependent effects mimicking PCP. These compounds are interesting examples of the structural requirements of molecules for PCP-mimetic activity, demonstrating that neither the piperidine nor the phenyl moieties are absolutely necessary for activity.
139

TABLE 1. Compounds |
generalizing to the PCP cue |
|
||||
|
|
|
|
|
|
|
|
Compound |
|
|
Structure |
|
ED50 |
|
|
PCP Cueing |
||||
|
PCP |
|
|
|
0.55 |
|
|
(±)Ketamine |
|
|
|
2.20 |
|
|
Tiletamine |
|
|
|
0.46 |
|
|
Dexoxadrol |
|
|
|
1.68 |
|
|
Levoxadrol |
|
|
|
>100 |
|
|
(-)2-MDP |
|
|
|
2.13 |
|
|
(+)2-MDP |
|
|
|
>10 |
|
|
± SKF 10047 |
|
|
|
2.74 |
|
NOTE: All compounds were administered SC |
30 |
minutes |
prior to |
a 15-minute test. |
ED50 values for compounds to mimic |
the |
effects |
produced |
by 1.78 mg/kg of |
PCP. |
|
|
|
|
When the animals were tested with dexoxadrol, PCP-mimetic activity was found to be about one-third that of PCP. This compound is of interest because its stereoisomer, levoxadrol, was found to be completely devoid of PCP-mimetic activity at doses as high as 100 mg/kg (table 1). Another example of the stereoselectivity of the PCP cue is seen with 2-methyl-3,3-diphenyl-3-propanolamine isomers (2-MDP). Consistent with the original findings by Tang et al. (1984), the (-) isomer of 2-MDP is a stereoselective PCP mimetic, with a potency about one-fourth that of PCP (table 1). SKF10,047, the prototypical sigma opiate receptor agonist, also engenders a dose-dependent generalization to the PCP cue. This compound has been extensively investigated for its discriminative properties (Shearman and Herz 1982; Teal and Holtzman 1980; Shannon 1983). and PCP-mimetic activity is exhibited stereospecifically by the dextro isomer (Brady et al. 1982).
140

TABLE 2. Structure-activity relationship for piperidine analogs mimicking the discriminative stimulus properties of PCP 1.78 mg/kg
|
Compound |
R |
|
ED50 |
|
|
Cueing |
|
|||
|
PCP |
|
|
0.55 |
|
|
CP-63,713-1 |
|
|
0.38 |
|
|
CP-63,986-1 |
|
<<3.2 |
|
|
|
CP-63,404-1 |
|
|
5.37 |
|
|
CP-63,631-1 |
|
1.65 |
racemlc |
|
|
|
|
0.33 |
R(+) |
|
|
|
|
>10 S(-) |
|
|
|
CP-63,402 |
|
|
11.5 |
|
|
CP-63,774-1 |
|
|
0.30 |
|
|
CP-63,579-1 |
|
|
1.04 |
|
|
|
|
|
|
|
In addition to the structurally diverse compounds described above, which can serve as PCP mimetics, analogs of PCP have also been examined for their ability to support PCP cueing. The most extensive work reported to date has been done in Harlan Shannon's laboratory (Shannon 1981; Cone et al. 1984; McQuinn et al. 1981) and has demonstrated (1) that the piperidine moiety is not required since the free primary amine is active; (2) modifications to the cyclohexyl ring greatly reduce or abolish activity; and (3) increasing the chain length to the phenyl group or replacement with naphthyl abolishes activity. In addition, table 2 shows that a number of piperidine modifications can be made and still retain PCP-mimetic activity.
141

In contrast to the generalization observed with PCP-like com-
pounds, |
a large number of agents that share similar pharmacologi- |
|
cal activities with PCP failed to mimic PCP's discriminative |
||
effects |
(table 3). |
Psychomotor stimulants, hallucinogens like THC |
and LSD, |
opiates, |
cholinergic and anticholinergic agents, as well |
as a diverse group of miscellaneous drugs, consistently produced vehicle choice.
TABLE 3. PCP discrimination. PCP 3.2 mg/kg vs. vehicle. Compounds failing to mimic PCP.
|
|
|
|
|
|
|
|
Percent PCP |
Response |
||
Compound |
|
|
|
|
Dose |
Range |
Choice |
Level |
|||
|
|
|
|
|
|
|
|
|
|
|
|
Amantadine |
|
|
|
|
10. |
- |
32. |
0 |
518 |
|
|
Methylphenidate |
|
|
|
|
3.2 |
- |
32. |
12 |
530 |
|
|
Cocaine |
|
|
|
|
3.2 |
- |
10. |
0 |
65 |
|
|
d-Amphetamine |
|
|
|
|
1.0 |
- |
1.78 |
0 |
140 |
|
|
Apomorphine |
|
|
|
|
.32 |
|
|
|
0 |
400 |
|
THC |
|
|
|
|
3.2 |
- |
10. |
12 |
455 |
|
|
Muscimol |
|
|
|
|
1.0 |
|
|
|
0 |
476 |
|
LSD |
|
|
|
|
.1 |
|
|
|
0 |
532 |
|
Yohimbine |
|
|
|
|
3.2 |
|
|
|
0 |
721 |
|
Morphine |
|
|
|
|
3.2 |
|
|
|
0 |
145 |
|
Ketocyclazoclne |
|
|
|
|
3.2 |
- |
56. |
25 |
144 |
|
|
Cyclazocine |
|
|
|
|
3.2 |
- |
32. |
12 |
357 |
|
|
Mecamylamine |
|
|
|
|
1.0 |
|
|
|
0 |
572 |
|
QNB |
|
|
|
|
1.0 |
- |
|
3.2 |
12 |
419 |
|
Nicotine |
|
|
|
|
1.0 |
|
|
|
0 |
691 |
|
Arecoline |
|
|
|
|
|
- |
|
3.2 |
12 |
360 |
|
Tacrine |
|
|
|
|
3.2 |
|
|
|
0 |
602 |
|
Physostigmine |
|
|
|
|
.1 |
- |
|
.32 |
12 |
136 |
|
Scopolamine |
|
|
|
|
.32 |
- |
|
1.0 |
0 |
141 |
|
Ditran |
|
|
|
|
10. |
|
|
|
0 |
250 |
|
Benrtropine |
|
|
|
|
3.2 |
- |
10. |
12 |
321 |
|
|
Pirbuterol |
|
|
|
|
10. |
|
|
|
0 |
309 |
|
Cyproheptadine |
|
|
|
|
3.2 |
|
- |
10. |
33 |
769 |
|
Althesin |
|
|
|
|
10. |
|
- |
32. |
25 |
233 |
|
Veratridine |
|
|
|
|
.001 |
- |
.32 |
12 |
8 3 |
|
|
Methaqualone |
|
|
|
|
32. |
|
-100. |
12 |
543 |
|
|
Navane |
|
|
|
|
10. |
|
- |
32. |
8 |
544 |
|
Pentoberbital |
|
|
|
|
10. |
|
|
|
0 |
391 |
|
Diazepam |
|
|
|
|
10. |
|
|
|
14 |
634 |
|
|
|
|
|
|
|
|
|||||
NOTE: A number |
of |
compounds |
failed to |
generalize to PCP when administered |
30 |
|
|||||
minutes |
prior |
to testing. |
N=at |
least 8 rats/treatment. Values are |
the |
||||||
highest |
generalization |
and |
lowest |
number |
of |
responses |
obtained over |
the |
|||
dose-range tested.
ATTEMPTS TO ANTAGONIZE PCP DISCRIMINATION
A search for compounds that might block the PCP cue found that many drugs failed to reverse PCP discrimination to the vehicle
level. Table 4 lists alphabetically the results obtained with such compounds over the dose range tested. A few agents,
142

TABLE 4. PCP discrimination. PCP 3.2 mg/kg vs. vehicle. Compounds failing to block PCP.
Compound |
|
|
|
|
|
|
|
Percent |
PCP |
Response |
|
|
|
|
|
Dose |
Range |
Choice |
|
Level |
|||
|
|
|
|
|
|
|
|
|
|
|
|
Alprazolam |
|
|
|
|
32. |
|
|
83 |
|
|
322 |
Amantadine |
|
|
|
|
10. |
|
|
100 |
|
|
382 |
Apomorphine |
|
|
|
|
.32 |
|
|
75 |
|
|
169 |
Baclofen |
|
|
|
|
1. |
- |
3.2 |
75 |
|
|
256 |
Caffeine |
|
|
|
|
56. |
|
|
87 |
|
|
253 |
Chlorpromazine |
|
|
|
.32 |
- |
10. |
50 |
|
|
46 |
|
Cinanserin |
|
|
|
|
3.2 |
|
|
100 |
|
|
326 |
Clonidine |
|
|
|
|
.032 |
|
87 |
|
|
172 |
|
ClozaDine |
|
|
|
|
1. |
- |
3.2 |
100 |
|
|
68 |
Cyproheptadine |
|
|
|
10. |
- |
10. |
83 |
|
|
198 |
|
Diazepam |
|
|
|
|
1. |
56 |
|
|
192 |
||
Diphenhydramine |
|
|
|
10. |
|
|
75 |
|
|
347 |
|
Dipyridamole |
|
|
|
|
32. |
|
|
92 |
|
|
457 |
Ditran |
|
|
|
|
1.0 |
|
|
67 |
|
|
130 |
Doxapram |
|
|
|
|
32. |
-178. |
50 |
|
|
615 |
|
Etazolate |
|
|
|
|
10. |
|
|
100 |
|
|
381 |
Haloperidol |
|
|
|
|
-056 |
|
100 |
|
|
296 |
|
|
|
|
|
|
;1 |
|
|
57 |
|
|
184 |
|
|
|
|
|
,178 |
|
56 |
|
|
64 |
|
|
|
|
|
|
.32 |
|
|
67 |
|
|
20 |
lmipramine |
|
|
|
|
10. |
|
|
75 |
|
|
248 |
Levamisol |
|
|
|
|
10. |
- |
32. |
91 |
|
|
252 |
Mecamylamine |
|
|
|
|
1.0 |
|
|
87 |
|
|
318 |
Methysergide |
|
|
|
|
32. |
|
|
75 |
|
|
240 |
Mezilamlne |
|
|
|
|
.1 |
|
|
50 |
|
|
338 |
|
|
|
|
|
.178 |
|
75 |
|
|
187 |
|
|
|
|
|
|
.178 |
|
87 |
|
|
193 |
|
|
|
|
|
|
.32 |
|
|
62 |
|
|
97 |
Morphine |
|
|
|
|
3.2 |
|
|
100 |
|
|
74 |
Naloxone |
|
|
|
|
10. |
-178. |
75 |
|
|
85 |
|
Naltrexone |
|
|
|
|
10. |
|
|
62 |
|
|
562 |
Nifedipine |
|
|
|
|
10. |
|
|
75 |
|
|
78 |
Nimodipine |
|
|
|
|
3.2 |
|
|
100 |
|
|
669 |
Oxolinicacid |
|
|
|
3.2 |
|
|
100 |
|
|
413 |
|
Pentylenetetrazol |
|
|
|
10; |
|
|
100 |
|
|
324 |
|
Phenltrone |
|
|
|
|
32. |
|
|
87 |
|
|
290 |
Phenytoin |
|
|
|
|
10. |
- |
32. |
100 |
|
|
288 |
Prazocine |
|
|
|
|
3.2 |
|
|
75 |
|
|
336 |
Propranolol |
|
|
|
|
10; |
|
|
71 |
|
|
291 |
QNB |
|
|
|
|
1.0 |
|
|
60 |
|
|
55 |
Scopolamine |
|
|
|
|
.1 |
|
|
87 |
|
|
441 |
Sulpiride |
|
|
|
|
32. |
|
|
87 |
|
|
478 |
Traiazolate |
|
|
|
|
32. |
|
|
92 |
|
|
510 |
TRH |
|
|
|
|
3.2 |
|
|
80 |
|
|
270 |
Vasopressin |
|
|
|
|
.5 |
- |
2.0 |
50 |
|
|
722 |
Yohimbine |
|
|
|
|
10. |
- |
32. |
50 |
|
|
238 |
|
|
|
|
|
|
|
|
||||
NOTE: Drugs |
which failed |
to reduce |
PCP |
discriminability |
below 50 |
percent. |
At |
||||
least 8 animals/treatment were tested. However, when response level was |
|||||||||||
suppressed below about 75 responses/15 minutes, a number of animals |
|
|
|||||||||
frequently |
failed to |
complete |
10 |
responses |
within |
15 minutes; their |
|
data |
|||
were |
not |
included. |
|
|
|
|
|
|
|
|
|
including doxapram, diazepam, haloperidol, vasopressin, and yohimbine, produced a partial antagonism to approximately the 50 percent PCP-choice level. Frequently, however, testing these agents at higher doses failed to reduce PCP discrimination further and/or resulted in complete disruption of responding. In contrast to the
143
absence of antagonism by these agents or by PCP analogs, we observed that potent, metabolically stable, adenosine analogs were capable of blocking completely the discriminative properties of PCP (Browne and Welch 1982). However, subsequent findings in our laboratory (Browne et al. 1983) indicated that the anti-PCP activity of adenosine analogs was most likely attributable to dispositional factors retarding PCP absorption into brain. It is of interest to note that, in contrast to reported antagonism of PCP's effects in other endpoints (e.g., locomotor activity), there has been no definitive report of an agent capable of completely reversing the discriminative properties produced by PCP. Since there is no known antidote to PCP intoxication in man, the discriminative stimulus assay may be an appropriate model for screening for such an agent.
In summary, the results presented here confirm and extend previous findings that PCP can serve as a discriminative stimulus, and that pharmacologically similar agents such as ketamine, dexoxadrol, and SKF-10,047 can mimic the PCP cue (Brady and Balster 1981; Brady et al. 1982; Herling et al. 1981; Holtzman 1980; Shannon 1981). It is known that PCP exerts a multiplicity of effects on brain monoaminergic and cholinergic systems (Ary and Kominskey 1980; Arora and Meltzer 1980; Ward and Trevor 1981). However, compounds that activate or attenuate activity of CNS noradrenergic, dopaminergic, serotonergic, cholinergic, GABAergic, opiate, or benzodiazepine systems all failed to mimic or block completely the discriminative effects of PCF. These results indicate that PCP-like agents probably exert their effects through unique neurochemical mechanisms distinctly different from other classes of agents. Indeed, it appears that PCP-like compounds may be acting on specific "PCP receptors" in brain (Vincent et al. 1979; Marwaha et al. 1981; Quirion et al. 1981; Zukin and Zukin 1979). Although good correlations between the ability of agents to support PCP cueing and their ability to displace labeled PCP from binding sites have been obtained (Zukin and Zukin 1981; Browne and Kozlowski, in preparation), it remains to be determined whether antagonists of PCP cueing and receptor binding can be eventually discovered.
REFERENCES
Arora, R.C., and Meltzer, H.Y. In vitro effect of phencyclidine and other psychomotor stimulants on serotonin uptake in human platelets. Life Sci 27:1607-1613, 1980.
Ary, T.E., and Kominskey, H.L. Basis of phencyclidine's ability to decrease the synaptosomal accumulation of [3H]-catechol- amines. Eur J Pharmacol 61:401-405, 1980.
Brady, K.T., and Balster, R.L. Discriminative stimulus properties of phencyclidine and five analogues in the squirrel monkey. Pharmacol Biochem Behav 14:213-218, 1981.
Brady, K.T.,; Balster, R.L.; and May, E.L. Stereoisomers of N- allylnormetazocine: Phencyclidine-like behavioral effects in squirrel monkeys and rats. Science 215:178-180, 1982.
144

Browne, |
R.G., and |
Fondren, B. |
-Endorphin and the narcotic cue. |
|
In: |
Colpaert, |
F.C., and Rosecrans, J.A., eds. Stimulus |
||
Properties of Drugs: Ten Years of Progress. Amsterdam: |
||||
Elsevier, 1978. |
pp. |
137-147. |
||
Browne, |
R.G., and |
Kozlowski, |
M.R. High affinity 3H-PCP binding |
|
sites predict discriminative stimulus properties in rats. In |
||||
preparation. |
|
|
|
|
Browne, |
R.G., and |
Welch, |
W.M. |
Stereoselective antagonism of phen- |
cyclidine's discriminative properties by adenosine receptor agonists. Science 217:1157-1159, 1982.
Browne, R.G.; Welch, W.M.; Kozlowski, M.R.; and Duthu, G. Antagonism of PCP discrimination by adenosine analogs. In: Kamenka, J.M.; Domino, E.F.; and Geneste, P., eds. Phencyclidine and Related Arylcyclohexylamines: Present and Future Applications. Ann Arbor: NPP Books, 1983. pp. 639-666.
Burns, R.S., and Lerner, S.E. Perspectives: Acute phencyclidine intoxication. Clin Toxicol 9:477-501, 1976.
Castellani, S., and Adams, P.M. Effects of dopaminergic drugs on phencyclidine-induced behavior in the rat. Neuropharmacology 20:371-374, 1981.
Castellani, S.; Adams, P.M.; and Giannini, A.J. Physostigmine treatment of acute phencyclidine intoxication. J Clin Psychiatry 43:10-11, 1982.
Colpaert, F.C.; Lal, H.; Niemegeers, C.J.E.; Lenaerts, F.M.; and Janssen, P.A.J. Investigations on drug produced and subjectively experienced discriminative stimuli. I. The fentanyl cue, a tool to investigate subjectively experienced drug actions. Life Sci 16:705-716, 1975.
Colpaert, F.C., and Rosecrans, J.A., eds. Stimulus Properties of Drugs: Ten Years of Progress. Amsterdam: Elsevier, 1978.
572 pp.
Cone, E.J.; McQuinn, R.L.; and Shannon, H.E. Structure-activity relationship studies of phencyclidine derivatives in rats. J Pharmacol Exp Ther 228:147-153, 1984.
Dorand, R.D. Phencyclidine ingestion: Therapy review. South Med
J 70:117-119, 1977. |
|
|
|
Freed, W.J.; Weinberger, D.R.; Bing, L.A.; and Wyatt, |
R.J. Neuro- |
||
pharmacological |
studies |
of phencyclidine (PCP)-induced behavior- |
|
al stimulation |
in mice. |
Psychopharmacology (Berlin) |
71:291-297, |
1980.
Garey, R.E.; McQuitty, S.; Tootle, D.; and Heath, R.G. The effects of apomorphine and haldol on PCP induced behavioral and motor abnormalities in the rat. Life Sci 26:277-284, 1980.
Herling, S.; Coale, E.H., Jr.; Hein, D.S.; Winger, G.; and Woods, J.H. Similarity of the discriminative stimulus effects of ketamine, cyclazocine, and dexoxadrol in the pigeon. Psychopharmacology (Berlin) 73:286-291. 1981.
Ho, B.T.; Richards, D.W.; and Chute, D.L., eds. Drug Discrimination and State Dependent Learning. New York: Academic Press, 1978. 392 pp.
Holtzman, S.G. Phencyclidine-like discriminative effects of opioids in the rat. J Pharmacol Exp Ther 214: 614-619, 1980.
145
Lal, H., ed. Advances in Behavioral Biology: Discriminative Stimulus Properties of Drugs. New York: Plenum Press, 1977. 239 pp.
Luby, E.D.; Cohen, B.D.; Rosenbaum, G.; Gottleib, J.J.; and
Kelly, R. Study of a new schizophrenomimetic drug--Sernyl. AMA Arch Neurol Psychiatry 81:363-369. 1959.
Luisada, P.V: The phencyclidine psychosis: Phenomenology and treatment. In: Petersen, R.C., and Stillman, R.C., eds. Phencyclidine (PCP) Abuse: An Appraisal. National Institute on Drug Abuse Research Monograph 21. DHEW Pub. No. (ADM) 73-728. Washington, D.C.: Supt. of Docs., U.S. Govt. Print. Off., 1978. pp. 241-253.
Luisada, P.V., and Brown, B.I. Clinical management of the phencyclidine psychosis. Clin Toxicol 9:531-545, 1976.
Marwaha, J.; Palmer, M.; Hoffer, B.; Freedman, R.; Rice, K.C.; Paul, S.; and Skolnick, P. Differential electrophysiological and behavioral responses to optically active derivatives of phencyclidine. Naunyn Schmiedebergs Arch Pharmacol 74:107-108, 1981.
McQuinn, R.L.; Cone, E.J.; Shannon, H.E.; and Su, T.-P. Phencyclidine: I. Structure-activity relationships of the cyclohexyl ring of phencyclidine. J Med Chem 24:1429-1432, 1981.
Quirion, R.; Rice, K.C.; Skolnick P.; Paul, S.; and Pert, C.B. Stereospecific displacement of [3Hl-phencyclidine (PCP) receptor binding by an enantiomeric pair of PCP analogs. Eur J Pharmacol 74:107-108, 1981.
Shannon, H.E. Evaluation of phencyclidine analogs on the basis of their discriminative stimulus properties in the rat. J Pharmacol Exp Ther 216:543-551, 1981.
Shannon, H.E. Pharmacological evaluation of N-allylnormetazocine (SKF10,047) on the basis of its discriminative stimulus properties in the rat. J Pharmacol Exp Ther 225:144-152, 1983.
Shearman, G.T., and Herz, A. Non-opioid psychotomimetic-like discriminative stimulus properties of N-allylnormetazocine (SKF 10,047) in the rat. Eur J Pharmacol 82:167-172, 1982.
Showalter, C.V., and Thornton, W.E. Clinical pharmacology of phencyclidine toxicity. Am J Psychiatry 134:1234-1238, 1977.
Stockard, J.J.; Werner, S.S.; Aalvers, J.A.; and Chiappa, K.H. Electroencephalographic findings in phencyclidine intoxication.
Arch Neurol 33:200-203, 1976. |
|
|
Stugeon, R.D.; Fessler, R.G.; London, |
S.F.; |
and Meltzer, H.Y. |
A comparison of the effects of neuroleptics on phencyclidine- |
||
induced behaviors in the rat. Eur J Pharmacol 76:37-53, 1981. |
||
Tang, A.H.; Cangelofi, A.A.; Code, R.A.; and Franklin, F.R. |
||
Phencyclidine-like behavioral effects of |
2-methyl-3,3-diphenyl- |
|
3-propanolamine (2-MDP). Pharmacol |
Biochem Behav 20:209-213, |
|
1984. |
|
|
Teal, J.J., and Holtzman, S.G. Discriminative stimulus effects of prototype opiate receptor agonists in monkeys. Eur J Pharmacol
68:1-10, |
1980. |
|
Thompson, T., and Pickens, R., |
eds. Stimulus Properties of Drugs. |
|
New York: |
Appleton-Century |
Crofts, 1971. |
146
Vincent, J.P.; Kartalovski, B.; Geneste, P.; Kamenka, J.M.; and Lazdunski, M. Interaction of phencyclidine ("angel dust") with a specific receptor in rat brain membranes. Proc Natl Acad Sci USA 76:4678-4682, 1979.
Ward, D., and Trevor, A. Phencyclidine-induced alteration in rat muscarinic cholinergic receptor regulation. Eur J Pharmacol 74:189-193, 1981.
Zukin, S.R., and Zukin, R.S. Specific [3H]phencyclidine binding in rat central nervous system. Proc Natl Acad Sci USA 76:53725376, 1979.
Zukin, S.R., and Zukin, R.S. Identification and characterization of 3H phencyclidine binding to specific brain receptor sites. In: Domino, E.F., ed. PCP (phencylidine): Historical and Current Perspectives. Ann Arbor: NPP Books, 1981.
AUTHOR
Ronald G. Browne, Ph.D.
Pfizer Central Research
Groton, CT 06340
147
